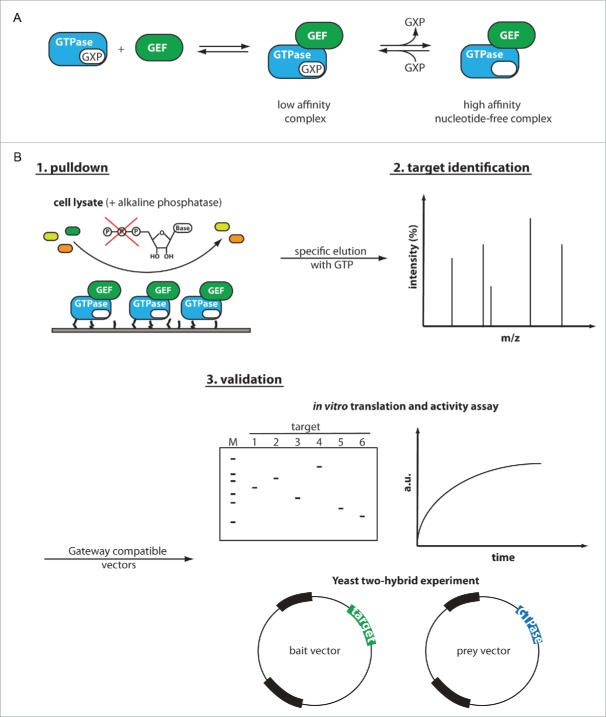
Figure 1.
Scheme for the specific enrichment and identification of unknown GEFs of small GTPases. (A) General reaction scheme of guanine nucleotide exchange factors for small GTPases. GEFs operate by transiting from a low affinity ternary GTPase:GXP:GEF intermediate to a high-affinity binary GTPase:GEF complex and back. In a first step, the GEF binds the GTPase:GXP complex with low affinity. GEF-mediated release of the nucleotide in the second step leads to a high affinity GEF:GTPase complex. In the reverse reaction, a different guanine nucleotide can bind, thereby completing the exchange reaction. (B) We envision to enrich the specific GEFs from cell lysates by exploiting the high-affinity of the intermediary nucleotide-free GTPase:GEF complex. The immobilized GTPase of interest is incubated with cell lysate containing its cognate GEF (green) among several other proteins (yellow and orange). Upon formation of a binary GTPase:GEF complex, the released nucleotide is degraded by exogenously added alkaline phosphatase to further stabilize the GTPase:GEF complex. After washing and removal of unbound proteins, the GEF can be specifically eluted by addition of GDP or GTP followed by subsequent identification via mass spectrometry. Since complex mixtures such as cell lysates may give rise to false positive targets, we designed the following procedures for GEF validation: I) An in vitro translation system coupled with an activity based GEF assay and II) a Y2H experiments with specifically designed GTPase mutants favoring GEF-interaction. The use of Gateway compatible vectors for both validation procedures greatly simplifies the cloning of different target proteins into expression vectors or vectors for Y2H experiments.