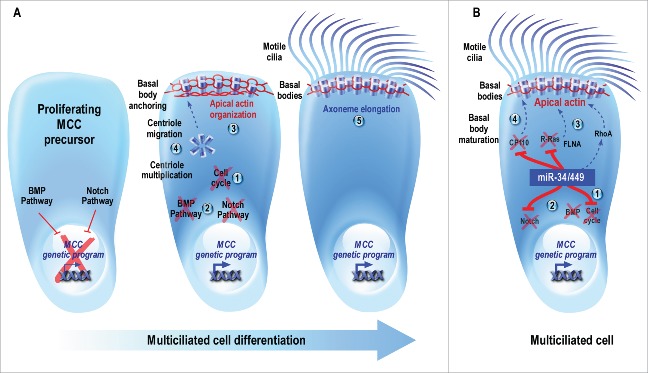
Figure 1.
(A) Schematic description of multiciliogenesis. Proliferating MCC precursors must undergo (1) cell cycle exit followed by (2) the inhibition of BMP and Notch pathways, 2 early events required for the entry into MCC differentiation. (3) In maturing MCCs, the apical actin cytoskeleton is reorganized into a dense cortical meshwork. (4) In addition, a massive multiplication of centrioles occurs, followed by their migration toward the apical membrane, where they mature and anchor to the actin meshwork to form ciliary organizing centers known as basal bodies. (5) Finally, MCC maturation is achieved through the elongation of axonemes from basal bodies to form multiple motile cilia. These motile cilia subsequently orient and beat in a coordinated manner to generate robust directional fluid flow at the surface of the epithelium. (B) Model illustrating the conserved roles of miR-34/449 during vertebrate MCC differentiation. The specific expression of miR-34/449 in immature MCCs allows (1) the exit from the cell cycle and (2) the entry into differentiation through the direct repression of the Notch pathway. As a result, the MCC genetic program is triggered. As miR-34/449 expression is itself repressed by the activation of the Notch signaling, these miRNAs accumulate in maturing MCCs, thus maintaining a double negative feedback loop. Then, miR-34/449 can regulate subsequent steps such as (3) the reorganization of the apical actin network, by directly repressing R-Ras, modulating the RhoA activity, and allowing the apical relocalization of FLNA, and (4) basal body maturation by directly repressing CP110. Plain lines indicate direct interactions; dotted lines identify pathways that may or may not be direct.