ABSTRACT
VE-cadherin-based cell-cell junctions form the major restrictive barrier of the endothelium to plasma proteins and blood cells. The function of VE-cadherin and the actin cytoskeleton are intimately linked. Vascular permeability factors and adherent leukocytes signal through small Rho GTPases to tightly regulate actin cytoskeletal rearrangements in order to open and re-assemble endothelial cell-cell junctions in a rapid and controlled manner. The Rho GTPases are activated by guanine nucleotide exchange factors (GEFs), conferring specificity and context-dependent control of cell-cell junctions. Although the molecular mechanisms that couple cadherins to actin filaments are beginning to be elucidated, specific stimulus-dependent regulation of the actin cytoskeleton at VE-cadherin-based junctions remains unexplained. Accumulating evidence has suggested that depending on the vascular permeability factor and on the subcellular localization of GEFs, cell-cell junction dynamics and organization are differentially regulated by one specific Rho GTPase. In this Commentary, we focus on new insights how the junctional actin cytoskeleton is specifically and locally regulated by Rho GTPases and GEFs in the endothelium.
Keywords: endothelium, GEF, GTPase, junction, VE-cadherin
Introduction
The endothelium covers the internal surface of blood vessels and controls the exchange of solutes, macromolecules and cells from blood to the underlying tissue. The adherens junction component vascular endothelial cadherin (VE-cadherin) is crucial to preserve endothelial barrier function. VE-cadherin regulates several aspects of endothelial biology, including permeability, leukocyte extravasation and blood vessel morphogenesis.1 The extracellular domain of VE-cadherin forms adhesive contacts between neighboring endothelial cells.2 VE-cadherin-based junctions are strengthened by the actin cytoskeleton, which interacts with cadherins through proteins of the catenin family.3 p120-Catenin binds directly to the membrane-proximal region of the cytoplasmic domain of VE-cadherin. β-Catenin and γ-catenin also associate directly with the cadherin cytoplasmic tail and serve as a scaffold to anchor α-catenin, which is a key mediator between cadherin and the actin cytoskeleton.4
Although the cadherin-catenin complex is commonly described as the ‘core’ VE-cadherin complex, many other proteins can associate, such as scaffolding proteins and cytoskeletal regulators.3,5 Some of these proteins, including vinculin,6-11 epithelial protein lost in neoplasm (EPLIN)12,13 α-actinin14 and afadin,15,16 have been found to bind to both α-catenin and actin and are therefore suggested to act as a link between the cadherin-catenin complex and actin. However, biochemical studies showed that a minimal cadherin-catenin complex consisting of E-cadherin, β-catenin and αE-catenin can directly bind to filamentous actin (F-actin). Strong interaction of this minimal cadherin-catenin complex to actin requires force.17 Interestingly, binding of vinculin to αE-catenin has also been demonstrated to be stabilized by tension.18,19 In endothelial cells, force exerted on cell-cell junctions was shown to recruit vinculin, which protected VE-cadherin junctions against opening.11 Together, these data suggest that tension on junctions may promote binding of cadherin/β-catenin as well as vinculin to α-catenin, resulting in their re-enforcement and growth. Conversely, increased actomyosin generated pulling force is important for opening of endothelial cell-cell junctions in response to permeability-inducing factors.20 By altering the magnitude and direction of the forces that are exerted on cell-cell junctions, actin cytoskeleton rearrangements can change the integrity of VE-cadherin-based cell-cell junctions.21 Thus, a finely balanced regulation of actin network organization, together with myosin-II activity, is needed to produce mechanical forces that drive assembly, maintenance and remodeling of adherens junctions (Fig. 1).
Figure 1.
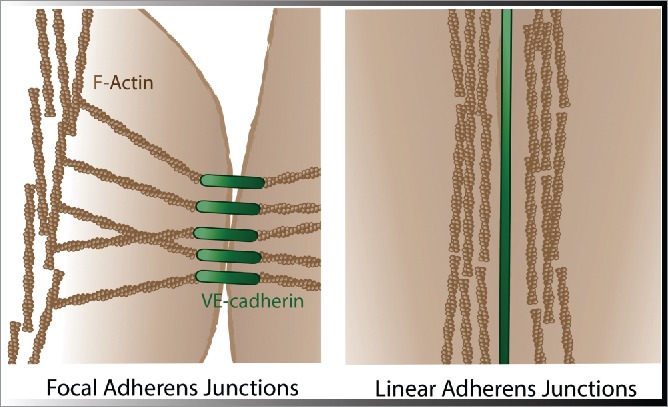
Organization of the actin cytoskeleton at endothelial cell-cell junctions. Left: Focal adherens junctions, or zipper-like junctions, are supported by radial actin bundles that exert tension on junction regions resulting in instable junctions and reduced integrity. Right: Linear junctions show the presence of cortical or so-called circumferential actin bundles that promote junction stability.
To achieve complete understanding of the tightly regulated spatial organization of cytoskeletal networks near junctions, we need to understand the dynamic signaling network in which Rho GTPases and their activators, GEFs, take part and how they impinge on actomyosin organization. We recently showed that binding of the GEF Trio to VE-cadherin is a crucial event to stabilize endothelial cell-cell junctions.22 Trio displays 2 GEF domains of distinct specificity, enabling activation of multiple Rho GTPases: Rac1, RhoG and RhoA.23,24 Our findings suggest that by activating Rac1 at junctions, Trio promotes the formation of cortical actin bundles adjacent to the junction, which is concomitant with the stabilization of cell-cell junctions and supports endothelial barrier function. Of note, the role of Rac1 in endothelial cell-cell adhesion seems contradictory in some occasions, as Rac1 has also been described to be involved in regulation of loss of VE-cadherin-based cell-cell contacts.25-27 This highlights the importance of the context-dependent and spatio-temporal regulation of Rac1 activity by GEFs at cell-cell junctions. Here, we will discuss how the local architecture of the actin cytoskeleton in proximity to cell-cell junctions is regulated by different GEFs and Rho GTPases and how this influences endothelial barrier function.
Actin at endothelial cell-cell junctions
F-actin characteristically concentrates at cadherin adhesion sites and can influence cell-cell junctions in different ways. The appearance of junctional actin differs between quiescent endothelium and endothelium that is challenged by permeability-inducing factors. In quiescent endothelium, junctions are aligned by thick cortical actin bundles. These cortical actin networks are regularly observed in close proximity to linear junctions, which show a continuous VE-cadherin labeling, and likely support the stabilization of cell-cell junctions.21 Challenging the endothelium, e.g., by permeability-inducing factors, induces cell-cell junction destabilization and is associated with the presence of radial contractile actin bundles. These radial F-actin bundles are perpendicular oriented and terminate at discontinuous cell-cell junctions. To discriminate between the distinct junction morphologies, we will refer to these junctions as focal adherens junctions (FAJs).3,11
Regulation of actin dynamics during endothelial cell-cell junction remodeling
Many kinds of dynamic actin-based structures and organizations exist, depending on the proteins that bind to it.28 Endothelial cells use distinct actin-based structures at different stages during the process of cell-cell junction formation, maintenance and remodeling, as was described in detail by Hoelzle and colleagues.29 Initial cell contact formation is driven by protruding lamellipodia of adjacent cells. Lamellipodia are generally initiated by the activated actin-related protein (ARP) 2/3 complex that induces actin nucleation and branching. Subsequently, retraction of lamellipodia leads to the formation of filopodia-like bridges connected through adherens junctions. This is accompanied by the recruitment of fascin and Myosin II activity. These bridges mature into stress fibers and are thought to strengthen the nascent junction by maintaining cells in close proximity to each other, which increases the chance of junction expansion.29 In contrast to endothelial cells which undergo multiple lamellipodia protrusion-retraction cycles during junction initiation,30 epithelial cells do not display a transition from lamellipodia to filopodia. Upon contact formation, cadherin molecules that were previously diffusing in the plasma membrane engage in homophilic interactions and form clusters. This homophilic ligation of cadherins triggers actin cytoskeleton rearrangements, driving contact expansion and stabilization.31 Thus, there is bidirectional interplay, and cadherin ligation has been demonstrated to recruit several actin regulators, including Rac1, Cdc42, Arp2/3, cortactin, N-WASP and Ena/VASP.32-36 37-40
Actin-driven membrane protrusions are not only required for nascent cell-cell junction formation, but are also described to be important for maintenance of integrity of mature junctions.41,42 At gaps between individual VE-cadherin clusters, lamellipodia arise and induce membrane overlap of adjacent cells. These structures were recently designated as junction-associated intermittent lamellipodia (JAIL).41 JAIL are described to facilitate lamellar-like VE-cadherin adhesion sites that are incorporated into the cell junctions upon JAIL retraction. By driving VE-cadherin dynamics, JAIL are suggested to allow adherens junctions to rapidly respond to inflammatory agents and growth factors.
Recently, Hultin and colleagues showed that the scaffolding protein AmotL2 links the VE-cadherin complex to MAGI-1 and actin filaments and in this way controls forces between neighboring cells via the cell-cell junctions.43 They additionally show that this interaction is required for the opening of the vascular lumen during vascular development. Yamamoto and colleagues revealed the importance of β1-integrins in the stabilization of cell-cell junctions.44 They demonstrate that integrin β1 is indispensable for proper localization of VE-cadherin, which is required for the formation of stable, non-leaky blood vessels. Thus, endothelial cell-cell junctions are highly dynamic and tightly regulated and there is interplay between cell-extracellular matrix adhesion and cell-cell adhesion within the blood vessel. Interestingly, Zovein and co-workers showed that β1-integrin is required for arterial endothelial cell polarity and lumen formation during development.45 This involves the polarity gene Par3 which is down regulated in β1-deficient endothelial cells and animals lacking endothelial β1 die at E9.5 and 10.46 Recently, Barry and colleagues showed the importance of VE-cadherin and the small GTPase Cdc42 in opening the vascular lumen.47 Hence, β1-integrins may be directly or indirecty linked to VE-cadherin during vessel development. If this interaction occurs during the maturation of vessels remains to be seen. But although focal adherent proteins such as vinculin 11 and also paxillin, FAK48 and zyxin49 have been detected in adherens junctions to co-localize with VE-cadherin under certain conditions (e.g., tension-induced or S-1-P), there is no complete understanding how integrins and VE-cadherin may collaborate or cross-talk to regulate the vascular barrier in matured junctions. In the next section, we focus more on the signaling pathways that control junctional actin remodeling and thereby determine its function.
Endothelial adherens junction control by Rho GTPases
Members of the Rho family of small GTPases are known to reorganize junction-associated actin and thereby regulate cell-cell adhesion. Small Rho GTPases cycle between an active GTP-bound state and an inactive GDP-bound state.50 In the activated state, Rho GTPases bind effector proteins to initiate a downstream response. Activation of Rho GTPases is mediated by guanine nucleotide-exchange factors (GEFs) that catalyze the exchange of GDP for GTP.51 Conversely, GTPase activating proteins (GAPs) enhance the intrinsic GTPase activity to inactivate the protein. Moreover, guanine-nucleotide-dissociation inhibitors (GDIs) sequester the GTPase within the cytosol and stabilize the GDP-bound state.52 RhoA and Rac1 are the so far best-characterized Rho GTPases.
The predominant effect of RhoA activation is formation of radial stress fibers and increased contractility, leading to enhanced vascular permeability. Effector proteins involved in RhoA-mediated signaling include the Rho-kinases (ROCKs), which further signal to Myosin Light Chain to stimulate actomyosin contraction.53 This has been shown to be essential for permeability induced by thrombin, VEGF, histamine and TNF-α.54-56 In contrast, RhoA activation in response to angiopoietin-1 has been described to result in cell-cell junction stabilization. This endothelial barrier-protective effect of RhoA in response to angiopoietin-1 is mediated via the Rho-effector mDia, inducing actin polymerization.57
Rac1 is often reported to be required for endothelial barrier maintenance and stabilization. Various barrier-stabilizing mediators activate Rac1, including sphingosine 1-phosphate (S1P) and angiopoietins.58-60 Barrier-protective cAMP signaling activates Rac1 indirectly via the Ras-GTPase Rap1.61,62 In addition, Rac1 activation downstream of thrombin promotes reassembly of cell-cell junctions during the endothelial barrier recovery phase.22,63 To promote (re)assembly of cell-cell junctions, Rac1 induces formation of lamellipodia protrusions. Using a novel FRET-based Rac1 biosensor we observed a global increase of Rac1 activation in these protrusions at the periphery of the endothelial cell. Rapidly after adjacent cells contacted each other, high Rac1 activity was measured locally at the nascent junction. This local Rac1 activity co-localized with α-catenin, one of the VE-cadherin complex members, and likely stabilized the nascent contacts by promoting the formation of cortical actin bundles.22 In line with this finding, a rapid increase in Rac1 activity upon VE-cadherin homotypic adhesion was detected biochemically, which was induced using beads coated with the VE-cadherin ectodomain. Thus, as was shown for E-, M- and N-cadherin,64-67 we showed that VE-cadherin can signal in an outside-in fashion to activate Rac1, providing a bidirectional feedback mechanism.22 Of note, ongoing local remodeling of cell-cell junctions is observed even in apparently stable endothelial monolayers. The involvement of Rac1 in maintenance of the endothelial barrier may, in part, reflect its requirement during cell-cell junction reassembly. In mature junctions, Rac1 activation was also recently demonstrated to reduce the rate of VE-cadherin dissociation, by using a photo-activatable probe to locally activate Rac1 at adherens junctions. Rac1 was shown to stabilize the VE-cadherin trans-interaction by counterbalancing RhoA-mediated actomyosin tension.68
Nevertheless, Rac1 activation also causes loss of cell-cell contacts upon stimulation of endothelial cells with VEGF, PAF and TNF-α, as well as clustering of vascular cell adhesion molecule (VCAM)-1.25,69-73 These data seem to be conflicting; however, they in fact underlie the importance of understanding the particular GEF-GTPase-effector complexes that are locally activated under resting and inflammatory conditions. We have summarized this in Figure 2. Thus, distinct spatiotemporal activation of these Rho GTPases can have different effects on endothelial junction organization and functional outcome. In many studies only global, but no local Rho-GTPase-mediated signaling events are detected. The effect of Rho-GTPase activity on cell-cell junctions also depends on the stimulus, demonstrating that the route of activation is critical.
Figure 2.
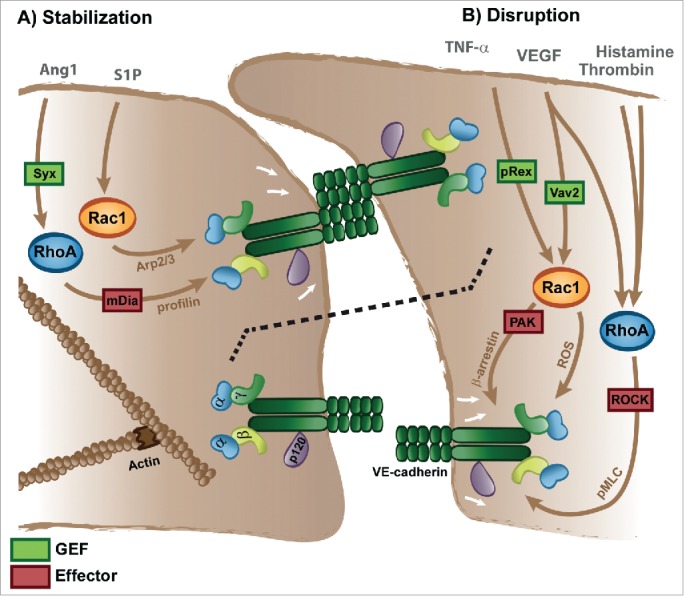
Regulation of VE-cadherin-based cell-cell adhesion by Rho-GTPases. Rho-GTPase signaling can either promote stabilization or disruption of cell-cell junctions. The effect of Rac1 and RhoA activity on cell-cell junctions depends on the stimulus. Different activation routes impact on distinct GEFs and effectors, specifying Rho-GTPase signaling. For details see main text.
In epithelial cells, Yamada and colleagues studied the localization of RhoA and Rac1 activation during junction formation using a Raichu FRET-based biosensor.74 They demonstrated that Rac1 activity is high at the periphery of contacting membranes, triggering initiation of cell-cell adhesion. In addition, RhoA activity at the contact edges was required to drive expansion and completion of the epithelial cell-cell junction.74 To what extent this study on spatiotemporal activation of Rho GTPases during epithelial cell contact formation also applies to VE-cadherin-based cell-cell junctions in endothelia remains to be explored.75
Another key player in endothelial cell-cell junction formation is the small GTPase Rap1.76 Rap1 can be artificially stimulated using the cAMP analog 8-pCPT-2′OMe-cAMP (also known as 007),77 which directly stimulates the Rap-GEF EPAC. Using this cAMP analog, Rap1 activation was shown to require VE-cadherin to improve the barrier function.76,78,79 Noda and coworkers showed that Rap1 activity promotes the stability of cell-cell junctions by reducing the mobility of VE-cadherin.80 More specifically, they showed that active Rap1 promotes the formation of circumferential actin bundles. In turn, VE-cadherin is connected to these bundles through α- and β-catenin. As a follow up, the same group showed that Rap1 activates Cdc42 through the Rho-GEF FGD5.81 This includes the local phosphorylation of myosin light chain (MLC) by the myotonic dystrophy-kinase related Cdc42-binding kinase (MRCK). Interestingly, a study by the Bos lab showed that Rap1 activation results in inhibition of RhoA activity, releasing tension from the junction region and thereby promoting stabilization of endothelial cell-cell junctions.82-84 Mechanistically they showed that the adapter protein Ras-interacting protein 1 (Rasip1) is involved in the formation of the endothelial barrier by forming a complex with Rap1 and promoting its activity.83 This results in the recruitment of ARHGAP29, a GAP that inactivates RhoA. Thus, by recruiting ARHGAP29, RhoA activity is believed to be locally inhibited, releasing junctional tension and thereby promoting cell-cell junction interaction.85,86
Endothelial Rho-GEFs and adherens junctions
GEFs outnumber their target Rho-GTPase by more than 3-fold.51 Therefore, different GEFs likely control different pools of the Rho GTPases, which directs local signaling pathways resulting in different outcomes.52 Thus far, a small number of studies aimed to unravel the precise role of different GEFs in the regulation of endothelial cell-cell junctions under resting and inflammatory conditions. An example of a RhoA-GEF localizing at cell-cell contacts is Syx, which promotes junction integrity by activating mDia. Of interest, VEGF-induced translocation of Syx away from cell-cell junctions resulted in junction disassembly, whereas angiopoietin-1 stabilized junctions by maintaining Syx at the junctions.87 The knowledge of Rac1-GEFs that participate in VE-cadherin signaling is also quite limited. Lampugnani and colleagues studied the effect of VE-cadherin expression on Rho-GTPase activity.88 Compared with VE-cadherin-deficient endothelial cells, VE-cadherin-reconstituted cells showed increased Rac1 activity and decreased activation of RhoA. This increase in Rac1 activity was suggested to be mediated by the GEF Tiam-1, since expression of VE-cadherin increased protein and mRNA levels of Tiam-1 and induced its localization at cell-cell junctions.88 Others showed that endothelial junction disruption in response to TNF-α was mediated by the GEF P-Rex1 through activation of Rac1. The P-Rex1-Rac1 signaling axis was described to induce generation of reactive oxygen species (ROS).89 ROS in turn increases vascular permeability by actin reorganization or by inactivating junction-associated phosphatases.90,91 Moreover, VEGF-induced Rac1 activation has been demonstrated to be regulated by the GEF Vav2, leading to generation of ROS and VE-cadherin internalization.25,92,93
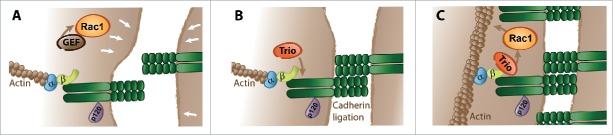
We recently showed that the multi-domain GEF Trio interacts with VE-cadherin. Trio is a member of the Dbl-family, containing 2 Dbl-homology-Pleckstrin-homoly (DH-PH) Rho-GEF units. In addition, Trio encodes an N-terminal putative lipid-transfer SEC14 domain, 9 spectrin-repeats, 2 SH3-domains, an Ig-like domain and a C-terminal serine/threonine kinase domain.94-101 The presence of these numerous domains indicates that Trio may function as an integrator of multiple signaling pathways. Trio is unique in that it can activate Rac1, RhoG and RhoA with its 2 separate GEF units.94-96 This diversity in Trio-targeted Rho GTPases seems contradictory since RhoA and Rac1 have apparent antagonistic effects. However, Rac1 and RhoA activity may be required at the same location but not at the same time. Our recent data allow us to propose a model that implicates the Rho-GEF Trio as an important regulator of endothelial adherens junctions (Fig. 3). During cell-cell junction (re-)assembly, initial cell-cell contact formation is promoted by Rac1 activity in the cell periphery, which occurs independent of Trio and induces lamellipodia protrusions. Subsequent trans-interactions between VE-cadherins from adjacent endothelial cells trigger outside-in signaling resulting in the recruitment of Trio to the intracellular tail of VE-cadherin and a local Trio-mediated activation of Rac1, specifically at the cell-cell junctions.22 This local Rac1 activity supports reorganization of the actin cytoskeleton in close proximity to the nascent contacts, promoting the switch of FAJs into linear junctions. In this model, Trio facilitates the transition from nascent-to-stable VE-cadherin-based cell-cell junctions and promotes the integrity of the endothelial monolayer. When cell-cell junctions are destabilized or physically disrupted, Trio dissociates from the VE-cadherin complex and is unable to promote Rac1 activity at cell-cell junction areas. Our data reveal an important and dynamic role for Trio to regulate the integrity of the endothelial barrier.
Figure 3.
Model of Trio-controlled endothelial adherens junction stabilization. (A) Cell-cell contact formation is driven by protruding lamellipodia of adjacent cells. These lamellipodia are initiated by activation of Rac1, which occurs independent of Trio. (B) Cadherin molecules that were previously diffusing in the plasma membrane engage in homophilic interactions and form clusters. VE-cadherin ligation triggers recruitment of Trio to the cell-cell junction. (C) Local Trio-mediated Rac1 activation rearranges the junctional actin cytoskeleton, leading to a transition from a nascent to stable cell-cell junction.
Although our findings indicate that junction formation is specifically associated with Trio-mediated Rac1 activation, our data do not exclude that Trio-mediated RhoA activation also can contribute to VE-cadherin-mediated cell-cell adhesion. In this regard, development of a specific inhibitor against the C-terminal domain of Trio, including its RhoA-GEF domain, may help to elucidate whether Trio may be capable to activate both Rac1 and RhoA in a spatially and temporally coordinated manner during the process of cell-cell junction remodeling. Of interest, Trio has been previously described by our group to control transendothelial migration of leukocytes by simultaneous induction of RhoG and Rac1 activation upon clustering of the adhesion receptor ICAM-1.102 Up to now, the mechanisms by which the individual domains of Trio are activated and by which distinct Rho GTPases are targeted are unclear.103
Concluding remarks
A particular Rho-GTPase can be part of signaling mechanisms that have opposing effects on endothelial cell-cell junctions. Local and time-dependent Rho-GTPase activity is crucial to ensure an optimal endothelial barrier function. Complexity of junction regulation is further increased by the bidirectional interplay between Rho GTPases and cadherins: Rho-GTPase activity can modulate cell-cell adhesion directly, but vice versa, engagement of cadherins upon cell-cell junction assembly can also stimulate or inhibit the activities of Rho GTPases.22,104,105 Hence, in addition to their structural role in mediating strong homotypic cell-cell adhesion, cadherins have the signaling capacity to regulate Rho-GTPase activity. A comprehensive view of the complex interplay between VE-cadherin, GEFs and Rho GTPases during diverse endothelial processes is still missing. A key issue for future research in this regard will be how Rho GTPases are localized to and activated at VE-cadherin-based cell-cell contacts. This will include study of GEFs in combination with the use of biosensors, enabling us to measure spatial and temporal activation of small GTPases. Eventually, understanding the molecular basis for vascular permeability, including signaling mechanisms regulating closure of endothelial junctions, will help to discover new therapeutic targets for diseases that involve excessive permeability or uncontrolled leukocyte infiltration into tissues.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
JDvB is supported by a Landsteiner Foundation for Blood Transfusion Research (LSBR) fellowship (grant #1028).
References
- [1].Dejana E, Vestweber D. The role of VE-cadherin in vascular morphogenesis and permeability control. Prog Mol Biol Transl Sci 2013; 116:119-44; PMID:23481193; http://dx.doi.org/ 10.1016/B978-0-12-394311-8.00006-6 [DOI] [PubMed] [Google Scholar]
- [2].Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E. The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin). J Cell Biol 1995; 129:203-17; PMID:7698986; http://dx.doi.org/ 10.1083/jcb.129.1.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Huveneers S, de Rooij J. Mechanosensitive systems at the cadherinGÇôF-actin interface. Journal of Cell Science 2013; 126:403-13; PMID:23524998; http://dx.doi.org/ 10.1242/jcs.109447 [DOI] [PubMed] [Google Scholar]
- [4].Navarro P, Caveda L, Breviario F, Mândoteanu I, Lampugnani MG, Dejana E. Catenin-dependent and -independent functions of vascular endothelial cadherin. J Biol Chem 1995; 270:30965-72; PMID:8537353; http://dx.doi.org/ 10.1074/jbc.270.36.21362 [DOI] [PubMed] [Google Scholar]
- [5].Bershadsky A. Magic touch: how does cell-cell adhesion trigger actin assembly? Trends Cell Biol. 2004; 14:589-93; PMID:15519846; http://dx.doi.org/ 10.1016/j.tcb.2004.09.009 [DOI] [PubMed] [Google Scholar]
- [6].Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol 2008; 10:1039-50; PMID:19160484; http://dx.doi.org/ 10.1038/ncb1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol 2010; 12:533-42; PMID:20453849; http://dx.doi.org/ 10.1038/ncb2055 [DOI] [PubMed] [Google Scholar]
- [8].Yao M, Qiu W, Liu R, Efremov AK, Cong P, Seddiki R, Payre M, Lim CT, Ladoux B, Mège RM, et al.. Force-dependent conformational switch of alpha-catenin controls vinculin binding. Nat Commun 2014; 5:4525; PMID:25077739 [DOI] [PubMed] [Google Scholar]
- [9].Hazan RB, Kang L, Whooley BP, Borgen PI. N-cadherin promotes adhesion between invasive breast cancer cells and the stroma. Cell Adhes Commun 1997; 4:399-411; PMID:9177902; http://dx.doi.org/ 10.3109/15419069709004457 [DOI] [PubMed] [Google Scholar]
- [10].Weiss EE, Kroemker M, Rudiger AH, Jockusch BM, Rudiger M. Vinculin is part of the cadherin-catenin junctional complex: complex formation between alpha-catenin and vinculin. J Cell Biol 1998; 141:755-64; PMID:9566974; http://dx.doi.org/ 10.1083/jcb.141.3.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Huveneers S, Oldenburg J, Spanjaard E, van der Krogt G, Grigoriev I, Akhmanova A, Rehmann H, de Rooij J. Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. The Journal of Cell Biology 2012; 196:641-52; PMID:22391038; http://dx.doi.org/ 10.1083/jcb.201108120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Abe J, Berk BC. Novel mechanisms of endothelial mechanotransduction. Arterioscler Thromb Vasc Biol 2014; 34:2378-86; PMID:25301843; http://dx.doi.org/ 10.1161/ATVBAHA.114.303428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chervin-Petinot A, Courcon M, Almagro S, Nicolas A, Grichine A, Grunwald D, Prandini MH, Huber P, Gulino-Debrac D. Epithelial protein lost in neoplasm (EPLIN) interacts with alpha-catenin and actin filaments in endothelial cells and stabilizes vascular capillary network in vitro. J Biol Chem 2012; 287:7556-72; PMID:22194609; http://dx.doi.org/ 10.1074/jbc.M111.328682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Knudsen KA, Soler AP, Johnson KR, Wheelock MJ. Interaction of alpha-actinin with the cadherin/catenin cell-cell adhesion complex via alpha-catenin. J Cell Biol 1995; 130:67-77; PMID:7790378; http://dx.doi.org/ 10.1083/jcb.130.1.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pokutta S, Drees F, Takai Y, Nelson WJ, Weis WI. Biochemical and structural definition of the l-afadin- and actin-binding sites of alpha-catenin. J Biol Chem 2002; 277:18868-74; PMID:11907041; http://dx.doi.org/ 10.1074/jbc.M201463200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tachibana K, Nakanishi H, Mandai K, Ozaki K, Ikeda W, Yamamoto Y, Nagafuchi A, Tsukita S, Takai Y. Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J Cell Biol 2000; 150:1161-76; PMID:10974003; http://dx.doi.org/ 10.1083/jcb.150.5.1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Buckley CD, Tan J, Anderson KL, Hanein D, Volkmann N, Weis WI, Nelson WJ, Dunn AR. Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science 2014; 346:1254211; PMID:25359979; http://dx.doi.org/ 10.1126/science.1254211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yao M, Qiu W, Liu R, Efremov AK, Cong P, Seddiki R, Payre M, Lim CT, Ladoux B, Mège RM, et al.. Force-dependent conformational switch of alpha-catenin controls vinculin binding. Nat Commun. 2014; 5:4525; PMID:25077739 [DOI] [PubMed] [Google Scholar]
- [19].Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol 2010; 12:533-42; PMID:20453849; http://dx.doi.org/ 10.1038/ncb2055 [DOI] [PubMed] [Google Scholar]
- [20].Moy AB, Van EJ, Bodmer J, Kamath J, Keese C, Giaever I, Shasby S, Shasby DM. Histamine and thrombin modulate endothelial focal adhesion through centripetal and centrifugal forces. J Clin Invest 1996; 97:1020-7; PMID:8613524; http://dx.doi.org/ 10.1172/JCI118493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Oldenburg J, Rooij J. Mechanical control of the endothelial barrier. Cell Tissue Res 2014;355:545-55; PMID:24519624; http://dx.doi.org/10652265 10.1007/s00441-013-1792-6 [DOI] [PubMed] [Google Scholar]
- [22].Timmerman I, Heemskerk N, Kroon J et al.. A local VE-cadherin/Trio-based signaling complex stabilizes endothelial junctions through Rac1. J Cell Sci 2015; 128:3514; http://dx.doi.org/ 10.1242/jcs.179424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Blangy A, Vignal E, Schmidt S, Debant A, Gauthier-Rouvière C, Fort P. TrioGEF1 controls Rac- and Cdc42-dependent cell structures through the direct activation of rhoG. J Cell Sci 2000; 113 (Pt 4):729-39; PMID:10652265 [DOI] [PubMed] [Google Scholar]
- [24].Debant A, Serra-Pages C, Seipel K, O'Brien S, Tang M, Park SH, Streuli M. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc Natl Acad Sci USA 1996; 93:5466-71; PMID:8643598; http://dx.doi.org/ 10.1073/pnas.93.11.5466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol 2006; 8:1223-34; PMID:17060906; http://dx.doi.org/ 10.1038/ncb1486 [DOI] [PubMed] [Google Scholar]
- [26].Spindler V, Schlegel N, Waschke J. Role of GTPases in control of microvascular permeability. Cardiovascular Research 2010; 87:243-53; PMID:20299335; http://dx.doi.org/ 10.1093/cvr/cvq086 [DOI] [PubMed] [Google Scholar]
- [27].van Wetering S, van Buul JD, Quik S, Mul FP, Anthony EC, ten Klooster JP, Collard JG, Hordijk PL. Reactive oxygen species mediate Rac-induced loss of cell-cell adhesion in primary human endothelial cells. J Cell Sci. 2002; 115:1837-46; PMID:11956315 [DOI] [PubMed] [Google Scholar]
- [28].Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science 2009; 326:1208-12; PMID:19965462; http://dx.doi.org/ 10.1126/science.1175862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hoelzle MK, Svitkina T. The cytoskeletal mechanisms of cellGÇôcell junction formation in endothelial cells. Molecular Biology of the Cell 2012; 23:310-23; PMID:22090347; http://dx.doi.org/ 10.1091/mbc.E11-08-0719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Millan J, Hewlett L, Glyn M, Toomre D, Clark P, Ridley AJ. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol 2006; 8:113-23; PMID:16429128; http://dx.doi.org/ 10.1038/ncb1356 [DOI] [PubMed] [Google Scholar]
- [31].Cavey M, Lecuit T. Molecular bases of cell-cell junctions stability and dynamics. Cold Spring Harb Perspect Biol 2009; 1:a002998; PMID:20066121; http://dx.doi.org/ 10.1101/cshperspect.a002998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Braga VM, Yap AS. The challenges of abundance: epithelial junctions and small GTPase signalling. Curr Opin Cell Biol 2005; 17:466-74; PMID:16112561; http://dx.doi.org/ 10.1016/j.ceb.2005.08.012 [DOI] [PubMed] [Google Scholar]
- [33].Shewan AM, Maddugoda M, Kraemer A, Stehbens SJ, Verma S, Kovacs EM, Yap AS. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol Biol Cell 2005; 16:4531-42; PMID:16030252; http://dx.doi.org/ 10.1091/mbc.E05-04-0330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Helwani FM, Kovacs EM, Paterson AD, Verma S, Ali RG, Fanning AS, Weed SA, Yap AS. Cortactin is necessary for E-cadherin-mediated contact formation and actin reorganization. J Cell Biol 2004; 164:899-910; PMID:15024035; http://dx.doi.org/ 10.1083/jcb.200309034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol Biol Cell 2005; 16:2636-50; PMID:15800060; http://dx.doi.org/ 10.1091/mbc.E05-01-0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell 2000; 100:209-19; PMID:10660044; http://dx.doi.org/ 10.1016/S0092-8674(00)81559-7 [DOI] [PubMed] [Google Scholar]
- [37].Bershadsky A. Magic touch: how does cell-cell adhesion trigger actin assembly? Trends Cell Biol. 2004; 14:589-93; PMID:15519846; http://dx.doi.org/ 10.1016/j.tcb.2004.09.009 [DOI] [PubMed] [Google Scholar]
- [38].Kovacs EM, Goodwin M, Ali RG, Paterson AD, Yap AS. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr Biol 2002; 12:379-82; PMID:11882288; http://dx.doi.org/ 10.1016/S0960-9822(02)00661-9 [DOI] [PubMed] [Google Scholar]
- [39].Nakagawa M, Fukata M, Yamaga M, Itoh N, Kaibuchi K. Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell-cell adhesion sites. J Cell Sci 2001; 114:1829-38; PMID:11329369 [DOI] [PubMed] [Google Scholar]
- [40].Noren NK, Niessen CM, Gumbiner BM, Burridge K. Cadherin engagement regulates Rho family GTPases. J Biol Chem 2001; 276:33305-8; PMID:11457821; http://dx.doi.org/ 10.1074/jbc.C100306200 [DOI] [PubMed] [Google Scholar]
- [41].Taha AA, Taha M, Seebach J, Schnittler HJ. ARP2/3-mediated junction-associated lamellipodia control VE-cadherinGÇôbased cell junction dynamics and maintain monolayer integrity. Molecular Biology of the Cell 2014; 25:245-56; PMID:24227887; http://dx.doi.org/ 10.1091/mbc.E13-07-0404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tang VW, Brieher WM. alpha-Actinin-4/FSGS1 is required for Arp2/3-dependent actin assembly at the adherens junction. J Cell Biol 2012; 196:115-30; PMID:22232703; http://dx.doi.org/ 10.1083/jcb.201103116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hultin S, Zheng Y, Mojallal M, Vertuani S, Gentili C, Balland M, Milloud R, Belting HG, Affolter M, Helker CS, et al.. AmotL2 links VE-cadherin to contractile actin fibres necessary for aortic lumen expansion. Nat Commun 2014; 5:3743; PMID:24806444; http://dx.doi.org/ 10.1038/ncomms4743 [DOI] [PubMed] [Google Scholar]
- [44].Yamamoto H, Ehling M, Kato K, Kanai K, van Lessen M, Frye M, Zeuschner D, Nakayama M, Vestweber D, Adams RH. Integrin beta1 controls VE-cadherin localization and blood vessel stability. Nat Commun 2015; 6:6429; PMID:25752958; http://dx.doi.org/ 10.1038/ncomms7429 [DOI] [PubMed] [Google Scholar]
- [45].Zovein AC, Luque A, Turlo KA, Hofmann JJ, Yee KM, Becker MS, Fassler R, Mellman I, Lane TF, Iruela-Arispe ML. Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev Cell 2010; 18:39-51; PMID:20152176; http://dx.doi.org/ 10.1016/j.devcel.2009.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lei L, Liu D, Huang Y, Jovin I, Shai SY, Kyriakides T, Ross RS, Giordano FJ. Endothelial expression of beta1 integrin is required for embryonic vascular patterning and postnatal vascular remodeling. Mol Cell Biol 2008; 28:794-802; PMID:17984225; http://dx.doi.org/ 10.1128/MCB.00443-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Barry DM, Xu K, Meadows SM, Zheng Y, Norden PR, Davis GE, Cleaver O. Cdc42 is required for cytoskeletal support of endothelial cell adhesion during blood vessel formation in mice. Development 2015; 142:3058-70; PMID:26253403; http://dx.doi.org/ 10.1242/dev.125260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sun X, Shikata Y, Wang L, Ohmori K, Watanabe N, Wada J, Shikata K, Birukov KG, Makino H, Jacobson JR, et al.. Enhanced interaction between focal adhesion and adherens junction proteins: involvement in sphingosine 1-phosphate-induced endothelial barrier enhancement. Microvasc Res 2009; 77:304-13; PMID:19323978; http://dx.doi.org/ 10.1016/j.mvr.2008.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Oldenburg J, van der Krogt G, Twiss F, Bongaarts A, Habani Y, Slotman JA, Houtsmuller A, Huveneers S, de Rooij J. VASP, zyxin and TES are tension-dependent members of Focal Adherens Junctions independent of the alpha-catenin-vinculin module. Sci Rep 2015; 5:17225; PMID:26611125; http://dx.doi.org/ 10.1038/srep17225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hall A. Rho GTPases and the actin cytoskeleton. Science 1998; 279:509-14; PMID:9438836; http://dx.doi.org/ 10.1126/science.279.5350.509 [DOI] [PubMed] [Google Scholar]
- [51].Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 2005; 6:167-80; PMID:15688002; http://dx.doi.org/ 10.1038/nrm1587 [DOI] [PubMed] [Google Scholar]
- [52].Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 2005; 21:247-69; PMID:16212495; http://dx.doi.org/ 10.1146/annurev.cellbio.21.020604.150721 [DOI] [PubMed] [Google Scholar]
- [53].van Hinsbergh VW, Nieuw Amerongen GP. Intracellular signalling involved in modulating human endothelial barrier function. J Anat 2002; 200:549-60; PMID:12162723; http://dx.doi.org/ 10.1046/j.1469-7580.2002.00060.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res 2000; 87:335-340; PMID:10948069; http://dx.doi.org/ 10.1161/01.RES.87.4.335 [DOI] [PubMed] [Google Scholar]
- [55].Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vascul Pharmacol 2002; 39:187-99; PMID:12747959; http://dx.doi.org/ 10.1016/S1537-1891(03)00008-9 [DOI] [PubMed] [Google Scholar]
- [56].Mikelis CM, Simaan M, Ando K, Fukuhara S, Sakurai A, Amornphimoltham P, Masedunskas A, Weigert R, Chavakis T, Adams RH, et al.. RhoA and ROCK mediate histamine-induced vascular leakage and anaphylactic shock. Nat Commun 2015; 6:6725; PMID:25857352; http://dx.doi.org/ 10.1038/ncomms7725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell 2008; 14:25-36; PMID:18194650; http://dx.doi.org/ 10.1016/j.devcel.2007.10.019 [DOI] [PubMed] [Google Scholar]
- [58].Felcht M, Luck R, Schering A, Seidel P, Srivastava K, Hu J, Bartol A, Kienast Y, Vettel C, Loos EK, et al.. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest 2012; 122:1991-2005; PMID:22585576; http://dx.doi.org/ 10.1172/JCI58832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].McVerry BJ, Garcia JG. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell Signal. 2005; 17:131-9; PMID:15494205; http://dx.doi.org/ 10.1016/j.cellsig.2004.08.006 [DOI] [PubMed] [Google Scholar]
- [60].David S, Ghosh CC, Mukherjee A, Parikh SM. Angiopoietin-1 requires IQ domain GTPase-activating protein 1 to activate Rac1 and promote endothelial barrier defense. Arterioscler Thromb Vasc Biol 2011; 31:2643-52; PMID:21885850; http://dx.doi.org/ 10.1161/ATVBAHA.111.233189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Spindler V, Peter D, Harms GS, Asan E, Waschke J. Ultrastructural analysis reveals cAMP-dependent enhancement of microvascular endothelial barrier functions via Rac1-mediated reorganization of intercellular junctions. Am J Pathol 2011; 178:2424-36; PMID:21457935; http://dx.doi.org/ 10.1016/j.ajpath.2011.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Baumer Y, Spindler V, Werthmann RC, Bunemann M, Waschke J. Role of Rac 1 and cAMP in endothelial barrier stabilization and thrombin-induced barrier breakdown. J Cell Physiol 2009; 220:716-26; PMID:19472214; http://dx.doi.org/ 10.1002/jcp.21819 [DOI] [PubMed] [Google Scholar]
- [63].Beckers CM, van Hinsbergh VW, van Nieuw Amerongen GP. Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thromb Haemost 2010; 103:40-55; PMID:20062930; http://dx.doi.org/ 10.1160/TH09-06-0403 [DOI] [PubMed] [Google Scholar]
- [64].Charrasse S, Comunale F, Fortier M, Portales-Casamar E, Debant A, Gauthier-Rouvière C. M-cadherin activates Rac1 GTPase through the Rho-GEF trio during myoblast fusion. Mol Biol Cell 2007; 18:1734-43; PMID:17332503; http://dx.doi.org/ 10.1091/mbc.E06-08-0766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].El Sayegh TY, Arora PD, Fan L, Laschinger CA, Greer PA, McCulloch CA, Kapus A. Phosphorylation of N-cadherin-associated cortactin by Fer kinase regulates N-cadherin mobility and intercellular adhesion strength. Mol Biol Cell 2005; 16:5514-27; PMID:16176974; http://dx.doi.org/ 10.1091/mbc.E05-05-0410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kovacs EM, Goodwin M, Ali RG, Paterson AD, Yap AS. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr Biol 2002; 12:379-82; PMID:11882288; http://dx.doi.org/ 10.1016/S0960-9822(02)00661-9 [DOI] [PubMed] [Google Scholar]
- [67].Perez TD, Tamada M, Sheetz MP, Nelson WJ. Immediate-early signaling induced by E-cadherin engagement and adhesion. J Biol Chem 2008; 283:5014-22; PMID:18089563; http://dx.doi.org/ 10.1074/jbc.M705209200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Daneshjou N, Sieracki N, van Nieuw Amerongen GP, Schwartz MA, Komarova YA, Malik AB, Conway DE. Rac1 functions as a reversible tension modulator to stabilize VE-cadherin trans-interaction. J Cell Biol 2015; 208:23-32; PMID:25559184; http://dx.doi.org/ 10.1083/jcb.201409108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gertzberg N, Neumann P, Rizzo V, Johnson A. NAD(P)H oxidase mediates the endothelial barrier dysfunction induced by TNF-alpha. Am J Physiol Lung Cell Mol Physiol 2004; 286:L37-48; PMID:12807699; http://dx.doi.org/ 10.1152/ajplung.00116.2003 [DOI] [PubMed] [Google Scholar]
- [70].Knezevic II, Predescu SA, Neamu RF, Gorovoy MS, Knezevic NM, Easington C, Malik AB, Predescu DN. Tiam1 and Rac1 are required for platelet-activating factor-induced endothelial junctional disassembly and increase in vascular permeability. J Biol Chem 2009; 284:5381-94; PMID:19095647; http://dx.doi.org/ 10.1074/jbc.M808958200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Monaghan-Benson E, Burridge K. The regulation of vascular endothelial growth factor-induced microvascular permeability requires Rac and reactive oxygen species. J Biol Chem 2009; 284:25602-11; PMID:19633358; http://dx.doi.org/ 10.1074/jbc.M109.009894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Naikawadi RP, Cheng N, Vogel SM, Qian F, Wu D, Malik AB, Ye RD. A critical role for phosphatidylinositol (3,4,5)-trisphosphate-dependent Rac exchanger 1 in endothelial junction disruption and vascular hyperpermeability. Circ Res 2012; 111:1517-27; PMID:22965143; http://dx.doi.org/ 10.1161/CIRCRESAHA.112.273078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].van Wetering S, van den BN, van Buul JD, Mul FP, Lommerse I, Mous R, ten Klooster JP, Zwaginga JJ, Hordijk PL. VCAM-1-mediated Rac signaling controls endothelial cell-cell contacts and leukocyte transmigration. Am J Physiol Cell Physiol 2003; 285:C343-52; PMID:12700137; http://dx.doi.org/ 10.1152/ajpcell.00048.2003 [DOI] [PubMed] [Google Scholar]
- [74].Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cellGÇôcell adhesion. The Journal of Cell Biology 2007; 178:517-27; PMID:17646397; http://dx.doi.org/ 10.1083/jcb.200701058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Yamazaki D, Oikawa T, Takenawa T. Rac-WAVE-mediated actin reorganization is required for organization and maintenance of cell-cell adhesion. J Cell Sci 2007; 120:86-100; PMID:17164293; http://dx.doi.org/ 10.1242/jcs.03311 [DOI] [PubMed] [Google Scholar]
- [76].Kooistra MR, Dube N, Bos JL. Rap1: a key regulator in cell-cell junction formation. J Cell Sci 2007; 120:17-22; PMID:17182900; http://dx.doi.org/ 10.1242/jcs.03306 [DOI] [PubMed] [Google Scholar]
- [77].Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Døskeland SO, Blank JL, Bos JL. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol 2002; 4:901-6; PMID:12402047; http://dx.doi.org/ 10.1038/ncb874 [DOI] [PubMed] [Google Scholar]
- [78].Lorenowicz MJ, Fernandez-Borja M, Kooistra MR, Bos JL, Hordijk PL. PKA and Epac1 regulate endothelial integrity and migration through parallel and independent pathways. Eur J Cell Biol 2008; 87:779-92; PMID:18635287; http://dx.doi.org/ 10.1016/j.ejcb.2008.05.004 [DOI] [PubMed] [Google Scholar]
- [79].Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 2005; 579:4966-72; PMID:16115630; http://dx.doi.org/ 10.1016/j.febslet.2005.07.080 [DOI] [PubMed] [Google Scholar]
- [80].Noda K, Zhang J, Fukuhara S, Kunimoto S, Yoshimura M, Mochizuki N. Vascular Endothelial-Cadherin Stabilizes at CellGÇôCell Junctions by Anchoring to Circumferential Actin Bundles through +¦- and +¦-Catenins in Cyclic AMP-Epac-Rap1 Signal-activated Endothelial Cells. Molecular Biology of the Cell 2010; 21:584-96; PMID:20032304; http://dx.doi.org/ 10.1091/mbc.E09-07-0580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ando K, Fukuhara S, Moriya T, Obara Y, Nakahata N, Mochizuki N. Rap1 potentiates endothelial cell junctions by spatially controlling myosin II activity and actin organization. The Journal of Cell Biology 2013; 202:901-16; PMID:24019534; http://dx.doi.org/ 10.1083/jcb.201301115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Post A, Pannekoek WJ, Ponsioen B, Vliem MJ, Bos JL. Rap1 Spatially Controls ArhGAP29 To Inhibit Rho Signaling during Endothelial Barrier Regulation. Mol Cell Biol 2015; 35:2495-502; PMID:25963656; http://dx.doi.org/ 10.1128/MCB.01453-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Post A, Pannekoek WJ, Ross SH, Verlaan I, Brouwer PM, Bos JL. Rasip1 mediates Rap1 regulation of Rho in endothelial barrier function through ArhGAP29. Proceedings of the National Academy of Sciences 2013; 110:11427-32; http://dx.doi.org/ 10.1073/pnas.1306595110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Pannekoek WJ, Post A, Bos JL. Rap1 signaling in endothelial barrier control. Cell Adh Migr 2014; 8:100-7; PMID:24714377; http://dx.doi.org/ 10.4161/cam.27352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Pannekoek WJ, Kooistra MR, Zwartkruis FJ, Bos JL. Cell-cell junction formation: the role of Rap1 and Rap1 guanine nucleotide exchange factors. Biochim Biophys Acta 2009; 1788:790-6; PMID:19159611; http://dx.doi.org/ 10.1016/j.bbamem.2008.12.010 [DOI] [PubMed] [Google Scholar]
- [86].Post A, Pannekoek WJ, Ponsioen B, Vliem MJ, Bos JL. Rap1 Spatially Controls ArhGAP29 To Inhibit Rho Signaling during Endothelial Barrier Regulation. Mol Cell Biol 2015; 35:2495-502; PMID:25963656; http://dx.doi.org/ 10.1128/MCB.01453-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ngok SP, Geyer R, Liu M, Kourtidis A, Agrawal S, Wu C, Seerapu HR, Lewis-Tuffin LJ, Moodie KL, Huveldt D, et al.. VEGF and Angiopoietin-1 exert opposing effects on cell junctions by regulating the Rho GEF Syx. J Cell Biol 2012; 199:1103-15; PMID:23253477; http://dx.doi.org/ 10.1083/jcb.201207009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lampugnani MG, Zanetti A, Breviario F, Balconi G, Orsenigo F, Corada M, Spagnuolo R, Betson M, Braga V, Dejana E. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Mol Biol Cell 2002; 13:1175-89; PMID:11950930; http://dx.doi.org/ 10.1091/mbc.01-07-0368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Naikawadi RP, Cheng N, Vogel SM, Qian F, Wu D, Malik AB, Ye RD. A critical role for phosphatidylinositol (3,4,5)-trisphosphate-dependent Rac exchanger 1 in endothelial junction disruption and vascular hyperpermeability. Circ Res 2012; 111:1517-27; PMID:22965143; http://dx.doi.org/ 10.1161/CIRCRESAHA.112.273078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Moldovan L, Moldovan NI, Sohn RH, Parikh SA, Goldschmidt-Clermont PJ. Redox changes of cultured endothelial cells and actin dynamics. Circ Res 2000; 86:549-57; PMID:10720417; http://dx.doi.org/ 10.1161/01.RES.86.5.549 [DOI] [PubMed] [Google Scholar]
- [91].Tonks NK. Redox redux: revisiting PTPs and the control of cell signaling. Cell 2005; 121:667-70; PMID:15935753; http://dx.doi.org/ 10.1016/j.cell.2005.05.016 [DOI] [PubMed] [Google Scholar]
- [92].Garrett TA, Van Buul JD, Burridge K. VEGF-induced Rac1 activation in endothelial cells is regulated by the guanine nucleotide exchange factor Vav2. Experimental Cell Research 2007; 313:3285-97; PMID:17686471; http://dx.doi.org/ 10.1016/j.yexcr.2007.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Monaghan-Benson E, Burridge K. The regulation of vascular endothelial growth factor-induced microvascular permeability requires Rac and reactive oxygen species. J Biol Chem 2009; 284:25602-11; PMID:19633358; http://dx.doi.org/ 10.1074/jbc.M109.009894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Bellanger JM, Lazaro JB, Diriong S, Fernandez A, Lamb N, Debant A. The two guanine nucleotide exchange factor domains of Trio link the Rac1 and the RhoA pathways in vivo. Oncogene 1998; 16:147-52; PMID:9464532; http://dx.doi.org/ 10.1038/sj.onc.1201532 [DOI] [PubMed] [Google Scholar]
- [95].Blangy A, Vignal E, Schmidt S, Debant A, Gauthier-Rouvière C, Fort P. TrioGEF1 controls Rac- and Cdc42-dependent cell structures through the direct activation of rhoG. J Cell Sci 2000; 113 (Pt 4):729-39; PMID:10652265 [DOI] [PubMed] [Google Scholar]
- [96].Debant A, Serra-Pages C, Seipel K, O'Brien S, Tang M, Park SH, Streuli M. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc Natl Acad Sci USA 1996; 93:5466-71; PMID:8643598; http://dx.doi.org/ 10.1073/pnas.93.11.5466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Medley QG, Serra-Pages C, Iannotti E, Seipel K, Tang M, O'Brien SP, Streuli M. The trio guanine nucleotide exchange factor is a RhoA target. Binding of RhoA to the trio immunoglobulin-like domain. J Biol Chem 2000; 275:36116-23; PMID:10948190; http://dx.doi.org/ 10.1074/jbc.M003775200 [DOI] [PubMed] [Google Scholar]
- [98].Medley QG, Buchbinder EG, Tachibana K, Ngo H, Serra-Pagès C, Streuli M. Signaling between focal adhesion kinase and trio. J Biol Chem 2003; 278:13265-70; PMID:12551902; http://dx.doi.org/ 10.1074/jbc.M300277200 [DOI] [PubMed] [Google Scholar]
- [99].Seipel K, Medley QG, Kedersha NL, Zhang XA, O'Brien SP, Serra-Pages C, Hemler ME, Streuli M. Trio amino-terminal guanine nucleotide exchange factor domain expression promotes actin cytoskeleton reorganization, cell migration and anchorage-independent cell growth. J Cell Sci 1999; 112 (Pt 12):1825-34; PMID:10341202 [DOI] [PubMed] [Google Scholar]
- [100].McPherson CE, Eipper BA, Mains RE. Multiple novel isoforms of Trio are expressed in the developing rat brain. Gene 2005; 347:125-35; PMID:15715966; http://dx.doi.org/ 10.1016/j.gene.2004.12.028 [DOI] [PubMed] [Google Scholar]
- [101].Saito K, Tautz L, Mustelin T. The lipid-binding SEC14 domain. Biochim Biophys Acta 2007; 1771:719-26; PMID:17428729; http://dx.doi.org/ 10.1016/j.bbalip.2007.02.010 [DOI] [PubMed] [Google Scholar]
- [102].van Rijssel J, Kroon J, Hoogenboezem M, van Alphen FP, de Jong RJ, Kostadinova E, Geerts D, Hordijk PL, van Buul JD. The Rho-GEF Trio controls leukocyte transendothelial migration by promoting docking structure formation. Mol Biol Cell 2012; 23(15):2831-44; PMID:22696684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].van Rijssel J, van Buul JD. The many faces of the guanine-nucleotide exchange factor trio. Cell Adh Migr 2012; 6:482-7; PMID:23076143; http://dx.doi.org/ 10.4161/cam.21418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Fukata M, Kaibuchi K. Rho-family GTPases in cadherin-mediated cell-cell adhesion. Nat Rev Mol Cell Biol 2001; 2:887-97; PMID:11733768; http://dx.doi.org/ 10.1038/35103068 [DOI] [PubMed] [Google Scholar]
- [105].Yap AS, Kovacs EM. Direct cadherin-activated cell signaling: a view from the plasma membrane. J Cell Biol 2003; 160:11-6; PMID:12507993; http://dx.doi.org/ 10.1083/jcb.200208156 [DOI] [PMC free article] [PubMed] [Google Scholar]