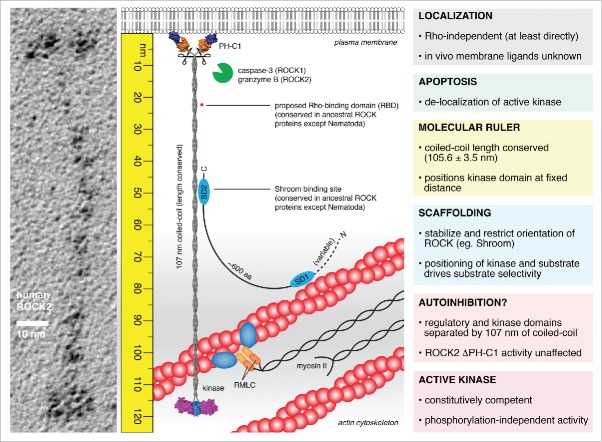
Figure 2.
The molecular ruler model of ROCK function. ROCK2 is an extended homodimer, 120 nm in length in which the kinase and regulatory domains are separated by 107 nm of parallel, semi-rigid coiled-coil. The kinase domains exist in a constitutively competent conformation mediated by the capped helix bundle (CHB) dimerization domain and an ordered, active conformation activation loop in the absence of phosphorylation. The regulatory domains exert no influence on catalytic activity. RhoA does not bind directly to either ROCK1 or ROCK2 in solution. The coiled-coil of ROCK is highly divergent in sequence, but remarkably conserved in length. Truncations in the coiled-coil, while retaining activity, cause the loss of actin stress fibers in cells, indicating the functional significance of its length. As such, the coiled-coil of ROCK bridges the kinase domains to a fixed distance from the plasma membrane. Scaffold proteins, such as Shroom, may bind to the coiled-coil of ROCK, stabilizing the orientation of the coiled-coil and perhaps restricting the positioning of the kinase domains. This leads to a model in which the phosphorylation of substrates by ROCK is governed by the spatial positioning of both kinase and substrate in the actin cytoskeleton. Proteolytic cleavage during apoptosis presumably liberates the active kinase domains, leading to delocalized activity, unregulated actomyosin contraction, and cell fragmentation.