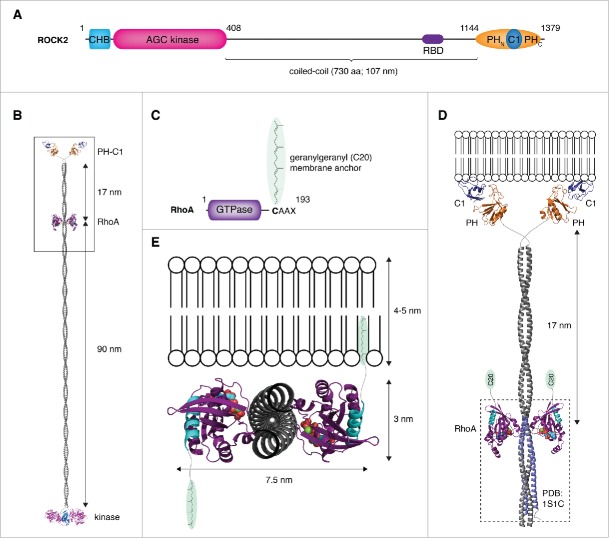
Figure 3.
Membrane binding by the ROCK:RhoA complex – a topological problem. (A) Schematic illustrating the primary domain composition and structure of ROCK. (B) Structural model of ROCK2 bound to RhoA. A model of ROCK2 was constructed by combining the high-resolution structures of the kinase and regulatory domains (PDB IDs: 2F2U, 2ROV, 2ROW) together with a modeled parallel coiled-coil, 107 nm in length. The complex of ROCK1:RhoA was docked onto the coiled-coil at a position corresponding to the location of the RBD in the primary sequence using the region of canonical coiled-coil in the structure (PDB ID: 1S1C) to which RhoA was observed to bind. In this model, RhoA binds to the coiled-coil at a position 90 nm distal to the kinase domains and 17 nm proximal to the membrane-binding domains. (C) RhoA is anchored in the membrane by a geranylgeranyl (C20) lipid anchor covalently attached to its C-terminus. (D) Docking the complex to a membrane such that the coiled-coil of ROCK is oriented perpendicular to the plane of the membrane results in the lipid anchors of RhoA being too far from the membrane. (E) Docking the complex to a membrane such that the coiled-coil of ROCK is parallel to the plane of the membrane results in the C-termini of RhoA (cyan) projecting in opposite directions. In this topology, only one molecule of RhoA would be capable of inserting its lipid anchor in the membrane.