Figure 1.
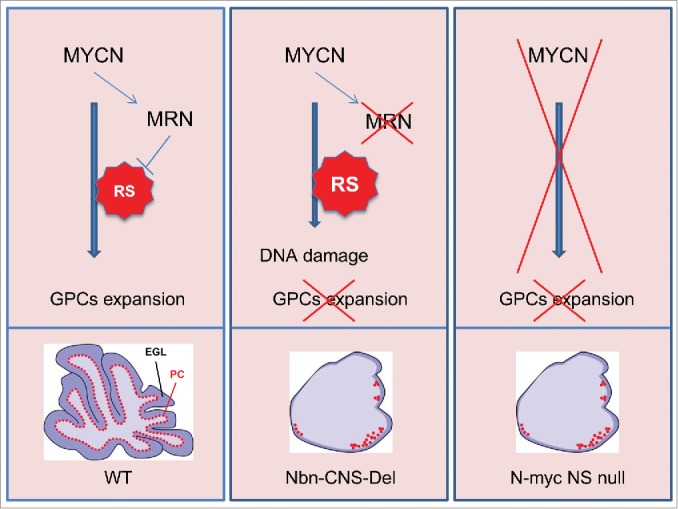
MYCN regulates expression of the MRE11/RAD50/NBS1 (MRN) complex to control the replication stress associated with granule cell progenitor (GCP) expansion during cerebellar development. MYC proteins promote DNA replication by activating the cell cycle machinery, by stimulating gene expression related to metabolic pathways, and by directly regulating replication origin firing. DNA replication under high MYC expression, how- 115 ever, generates replication stress. This has been attributed to (1) a higher density of replicating origins; (2) increased replication fork stalling or collapse; (3) collisions between replication and transcriptional apparatuses; and (4) CDC45 recruitment on replication origins. During the expansion wave of cerebellar 120 GCPs, the high expression of MYCN induces RS and increased expression of the MRN complex, whose function is strictly required to prevent the deleterious effects of RS on DNA integrity. Indeed, MRN inhibition at this level leads to DNA damage accumulation and GCP cell death. According to this model, the 125 cerebellum of the Nbn-CNS-Del KO mouse is characterized by extremely reduced foliation, minimal expansion of GCPs, and reduction and mislocalization of the Purkinje cells (PC). These features are strikingly similar to those observed in the nervous system-restricted N-myc KO mouse.
