Table 2.
Kallikrein inhibitors with structural diversity and varying potency reported in the literature
| Organization | Drug Name | Structure | Highest Phase | MOA | Condition | Basic Patent |
|---|---|---|---|---|---|---|
| Fovea,Cubist: Dyax | DX-88 |

|
Recommended Approal | Plasma Kallikrfein inhibitor | Surgery, arterial coronary; Angioedema, hereditary; Edema, macular | US 2006183771; WO 2006036860 |
| Showa Denko | PKSI-527 |
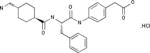
|
Preclinical | Plasma Kallikrein inhibitor | Thrombosis | EP 0217286 |
| Daiichi Sankyo |
|
Biological Testing | Plasma Kallikrein inhibitor | Pancreatitis | JP 1992159261 | |
| Novo Nordisk |

|
Biological Testing | Plasma Kallikrein inhibitor | Pancreatitis | US 5373090 | |
| Bristol-Meyers Squibb |
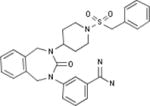
|
Biological Testing | Kallikrein 1 (Plasma Kallikrein) Inhibitors; Inhibitors of Blood Coagulation Pathways | Thrombosis | WO 1997038984 | |
| Celera |
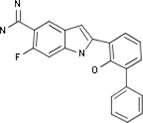
|
Biological Testing | Plasmin Inhibitors; Urokinase (u-PA) Inhibitors; Kallikrein 1 (Plasma Kallikrein) Inhibitors | Thrombosis | WO 2000035886 |
Source: Prous Integrity
