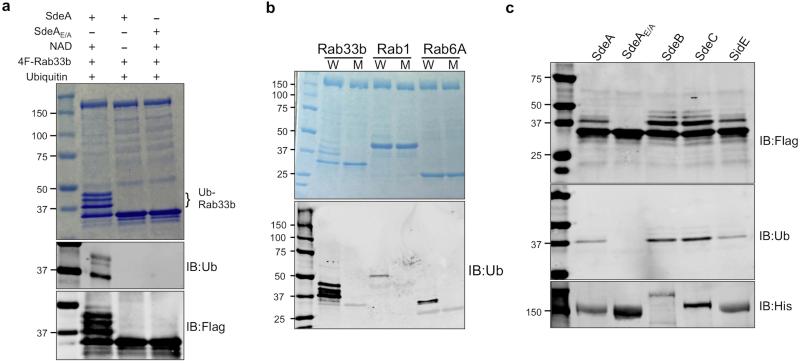
Extended Data Figure 4. The activity of EDTA-dialyzed SdeA and other members of the SidE family.
a, SdeA or SdeAE/A dialyzed against a buffer containing 10 mM EDTA was used for in vitro ubiquitination of Rab33b. Reactions were allowed to proceed for 2 h at 37°C. Samples resolved by SDS-PAGE were detected by Coomassie staining (upper panel), by immunoblotting with antibodies specific for ubiquitin (middle panel) or for the Flag tag (lower panel). Note that the addition of exogenous NAD is sufficient to allow SdeA-mediated ubiquitination of Rab33b (lane 2). b, In vitro Ubiquitination of Rabs by SdeA. Reactions containing indicated proteins and NAD were allowed to proceed for 2 h at 37°C. After SDS-PAGE, ubiquitinated proteins were detected by staining 50% of the reactions resolved by SDS-PAGE with Coomassie (upper panel) or by immunoblotting with antibodies specific for ubiquitin (lower panel). Similar results were obtained from two experiments. c, In vitro ubiquitination of Rab33b by SidE, SdeB1-1751 and SdeC. Indicated testing proteins were incubated with NAD, ubiquitin and Flag-Rab33b for 2 h at 37°C. Proteins resolved by SDS-PAGE were detected by antibodies specific for Flag (upper panel) or for ubiquitin (middle panel). His6-tagged SdeA, SdeB1-1751 and SdeC and SdeAE/A used in the reactions were probed 10% of the proteins with an antibody against His (lower panel). Similar results were obtained from two independent experiments. a, b, c, Uncropped blots are shown in Supplementary Fig. 1.