Figure 3.
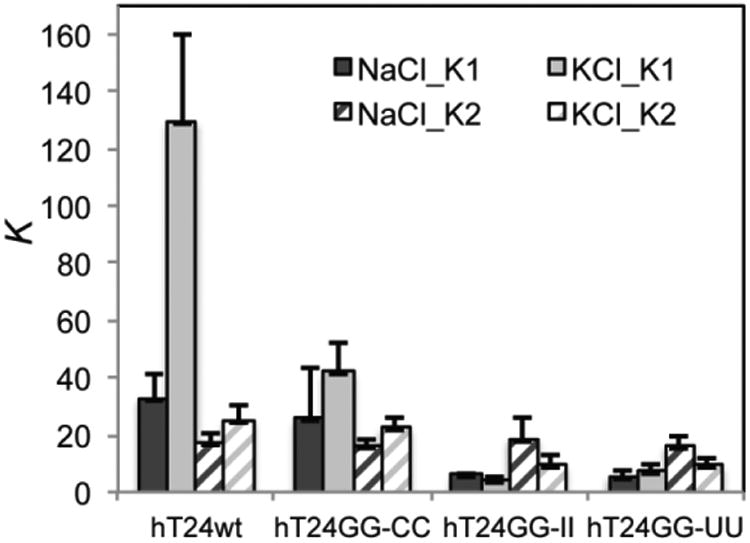
Different G-quadruplex structures alter the equilibrium binding affinity of POT1-TPP1. Sequential POT1-TPP1 protein binding reactions were evaluated by EMSA (Supplemental Figures 3-6) as described in Figure 2. Binding of POT1-TPP1 protein was evaluated with four DNA substrates in either 150 mM NaCl or 150 mM KCl. The affinity of the first protein binding event to G-quadruplex structures formed by hT24wt DNA in KCl is significantly hindered compared to all other DNA substrates analyzed, including G-quadruplex structures formed by hT24wt in NaCl. In contrast, binding affinities of subsequent protein binding events was similar for all DNA substrates in NaCl or KCl. All data represent results obtained from three independent experiments.
