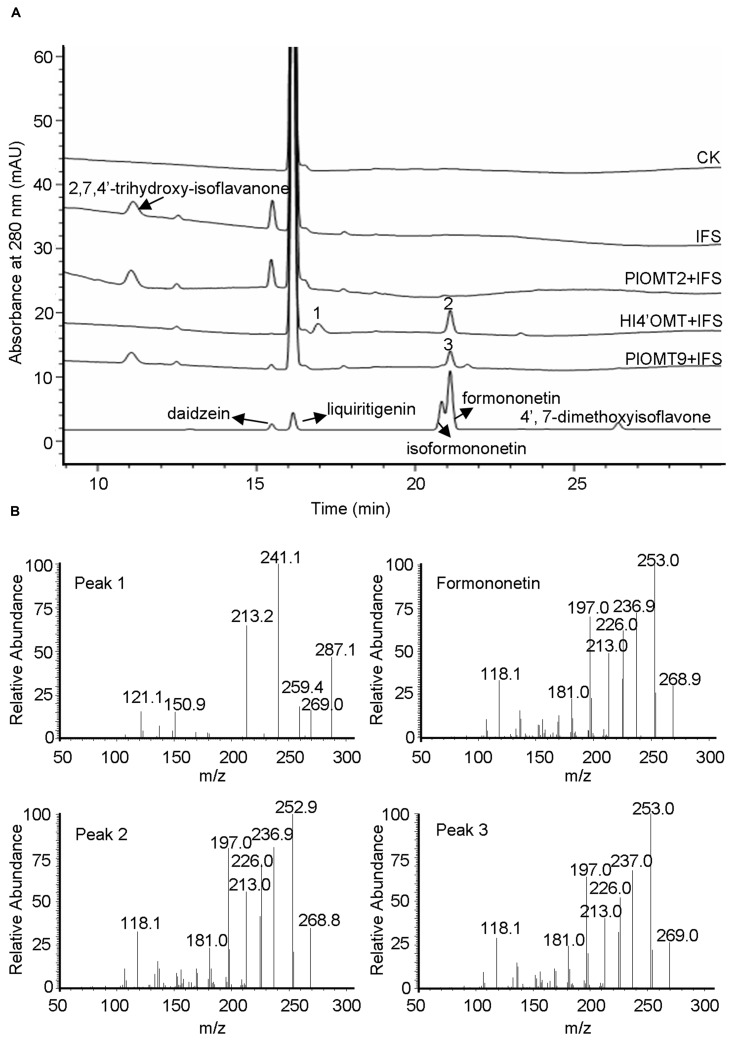
FIGURE 4.
LC-MS analysis of the products from the in vitro assays of the purified recombinant PlOMT2, PlOMT9 or HI4′OMT with 2,7,4′-trihydroxy-isoflavanone. (A) HPLC profiles were shown for the production of 2,7-dihydroxy-4′-methoxy-isoflavanone (peak 1) and formononetin (peak 2) in the reaction with HI4′OMT, formononetin (peak 3) in the reaction with PlOMT9, and no enzymatic products in the reactions with PlOMT2; (B) the mass spectra of peaks 1–3 and formononetin standard; The substrate 2,7,4′-trihydroxy-isoflavanone was prepared by the incubation of the yeast microsome expressing P. lobata IFS (GenBank accession number KC202929) with a racemic mixture of 2R/S-liquiritigenin and NADPH; The collision energy in the LC-MS analysis is 15 V for 2,7-dihydroxy-4′-methoxy-isoflavanone and 30 V for formononetin.