Figure 2.
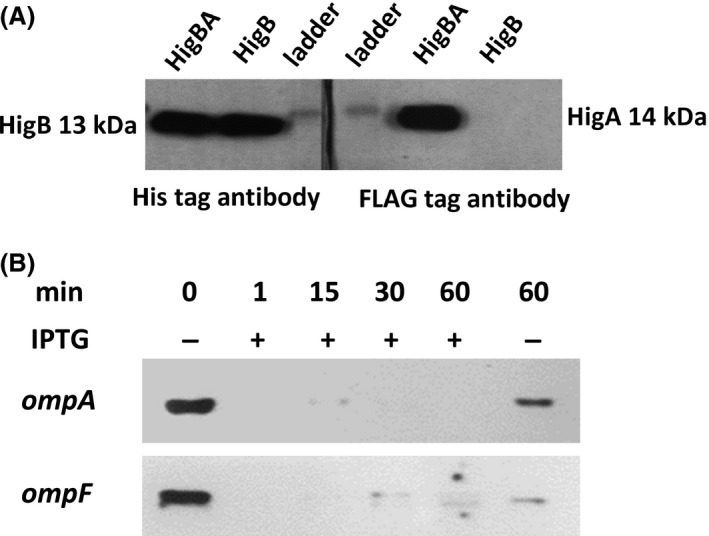
Western blot of HigB/HigA showing HigA binds to HigB and HigB degrades ompA and ompF mRNA in vivo. Escherichia coli TG1/pCA24N(His‐higB) and E. coli TG1/pCA24N(His‐higB‐higA‐FLAG) were grown in LB medium at 37°C at an initial turbidity of 0.05 at 600 nm, then 0.1 mmol/L of IPTG was added to produce HigB and HigA for 5 h. (A) The toxin HigB was tagged with six histidines, and the antitoxin HigA was tagged with the FLAG octapeptide. After producing both proteins via the E. coli TG1/pCA24N(His‐higB‐higA‐FLAG) strain, HigA binding to HigB was checked based on binding of HigB to the nickel column via its His tag. After purification of HigB, both HigB (left hand panel, using a His antibody) and HigA (right hand panel, using a FLAG antibody) from the HigB/HigA complex (HigBA) were detected independently in a denaturing gel. HigB production from the E. coli TG1/pCA24N(His‐higB) strain served as a positive control for HigB detection via the His antibody (left hand panel) as well as a negative control for the absence of HigA detection with the FLAG antibody (right hand panel). (B) RNA was isolated after higB was induced with 0.5 mmol/L of IPTG using the E. coli TG1/pCA24N(His‐higB) strain for 0, 1, 15, 30, and 60 min. Northern blot analysis was performed for ompA and ompF RNA detection.
