Abstract
Oxidative stress plays critical roles in the pathogenesis of diabetes, hypertension, and atherosclerosis; some authors reported that fat accumulation correlates to systemic oxidative stress in human and mice, but cellular redox environment effect on lipid accumulation is still unclear. In our laboratory we used mouse embryonic fibroblasts (undifferentiated cells: CC), which are capable of differentiating into mature adipocytes (differentiated cells: DC) and accumulate lipids, as obesity model. Here we analyzed the role of the well-known antioxidant and glutathione precursor N-acetylcysteine (NAC) in cellular MAPK modulation and lipid accumulation. We evaluated the effect of NAC on the adipogenic differentiation pathway using different doses: 0.01, 0.1, 1 and 5 mM; no toxic doses in these cells. A dose of 5 mM NAC [DCN-5] provoked a significant decrease in triglyceride accumulation (72±10 [DCN-5] vs 169±15 [DC], p<0.01), as well in Oil Red O stained neutral lipid content (120±2 [DCN-5] vs 139±12 [DC], p<0.01). Molecular mechanisms responsible for adipogenic differentiation involve increase of the expression of phosphoERK½ and phosphoJNK, 5 mM NAC treatment inhibited both pERK½ and pJNK protein levels. We also evaluated the mitotic clonal expansion (MCE) which takes place during adipogenesis and observed an increase in DC at a rate of 1.5 cells number compared to CC at day 2, whereas the highest doses of NAC significantly inhibited MCE. Our results suggest that NAC inhibits lipid accumulation and the MAPK phosphorylation in mouse embryonic fibroblasts during adipogenic differentiation and further contribute to probe the importance of cellular redox environment in adipogenesis.
Keywords: N-acetylcysteine, Antioxidants, MEF, Adipogenesis, Kinases, Lipids
Graphical abstract
Highlights
-
•
NAC, up to 5 mM, is not toxic in adipocytes obtained from mouse embryonic fibroblasts.
-
•
NAC inhibited phosphorylation of ERK½ and JNK in adipogenic differentiation.
-
•
NAC inhibited mitotic clonal expansion in adipogenic differentiation.
-
•
NAC inhibited triglyceride and lipid accumulation in mouse embryonic adipocytes.
1. Introduction
Research investigating redox regulation has received a large amount of attention due to the role of oxidative stress in several diseases. Excessive production of reactive oxygen species (ROS) induces oxidative stress in cells, but non-toxic levels of ROS have been described in relation to intracellular signal transduction, thereby regulating fundamental cell behaviors such as proliferation and differentiation [1]. However, ROS have a very short half-life, and their cellular levels are very difficult to reproduce. Therefore, the effect of ROS can be difficult to measure. An alternative strategy to achieve this goal is to evaluate the effects of antioxidants in a systemic study. New adipocytes could develop from precursor such 3T3-L1 fibroblasts or mouse embryonic fibroblasts (MEF). We have previously shown that antioxidant N-acetylcysteine (NAC) inhibits adipogenic differentiation in the 3T3-L1 cell line [2], [3]. Here, we explored this antioxidant effects on primary cultures from MEF because 3T3-L1 are committed cells.
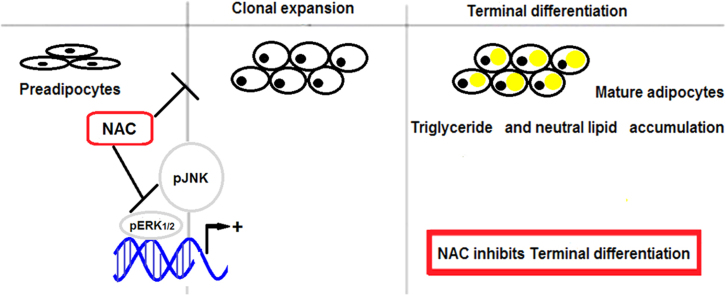
The molecular mechanisms that are responsible for the adipogenic differentiation involve regulation of the expression of MAPKs (Mitogen-Activated Protein Kinases) such as phospho-ERK (p ERK½) and phospho-JNK (pJNK). This regulation leads to terminal differentiation and accumulation of triglycerides (Tg) in the adipocytes and as a consequence, the potential to develop obesity [4], [5]. As for the role of ERK½ in differentiation process, it is involved in an initial proliferation called mitotic clonal expansion (MCE) that takes place during the two first days of adipogenesis [6]. The signaling pathways that involve JNK are strongly responsive to redox regulation. Thus, an exploration of the molecular regulation of ERK½ and JNK MAPKs during adipogenesis is important for understanding cellular differentiation. Of particular interest is the modulation that occurs during an antioxidant treatment that inhibits the accumulation of triglycerides, which would be the final event in the differentiation of preadipocytes. Questions such as “how does the activation of mitogen-activated protein kinase (MAPK) modules in response to different extracellular inputs lead to distinct effects in cellular metabolism?” [7] could be answered using this strategy.
The use of NAC as a regulator of the adipogenic process is under discussion [2], [3], [8], [9]. In the present study, our aim is to evaluate the relationship between the accumulation of lipids and MAPK during MEF cellular differentiation through treatment with the antioxidant NAC.
2. Materials and methods
2.1. Isolation of mouse embryonic fibroblasts (MEF)
Mouse embryonic fibroblasts (MEFs) were prepared from CF-1 mouse embryos at day 14 of gestation, by culture of small tissue explants as previously described [10]. Briefly, the embryos were removed from the uterus and washed with PBS. Once the head and red organs were dissected, the embryonic tissue was washed with PBS and finely minced using a sterile razor blade until the tissue could be handled with a pipette. After that, trypsin-EDTA was added and the sample was incubated for 30 min at 37 °C. Trypsin was inactivated by adding Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 25 mM glucose and 10% fetal bovine serum (FBS). Cells were centrifuged at 300 g for 5 min; the pellet was plated in culture bottles with complete media (DMEM plus 25 mM glucose and 10% FBS). The outgrowing primary cell population was passaged by trypsinization at ratio of 1:3 upon confluency and continuously cultured in complete media to favor growth of fibroblastic cells.
2.2. MEF adipocyte differentiation
MEF were first cultured in MDI medium (0.5 mM 3-isobutyl-1-methyl xanthine, 0.1 μM dexamethasone, and 2 μM insulin) for 72 h. They were then transferred to fresh DMEM (25 mM glucose; 10% FBS) supplemented with 2 μM insulin and incubated for three days. The cells were then cultured in fresh complete media for the remainder of the experiment. Day 0 of differentiation was defined as the time at which the cells were first introduced to MDI medium. At day 10, 70–80% of cells dramatically increased their triglyceride (Tg) content, thereby generating refractive droplets that were easily observed by microscopy or Oil Red O staining. MDI-treated MEF were considered differentiating cells (DC), and vehicle-treated MEF were considered control cells (CC).
NAC was added to the MDI medium at day 0 of differentiation and maintained in the medium throughout the remainder of the experiment; these cells were considered NAC-treated differentiating cells (DCN).
2.3. Oil Red O staining
Cell monolayers were washed three times with PBS and then fixed with 4% formaldehyde in PBS, at 4 °C for 30 min. A stock solution of 0.4% Oil Red O (SIGMA) in isopropanol was prepared. To perform the assay, the dye was diluted with water, filtered and added to the fixed cells. Oil Red O staining was developed at room temperature for 30 min. Cells were then washed with water and, the stained lipids droplets in the cells were visualized and photographed. After that, the dye was extracted with isopropanol and its absorbance was determined at 510 nm to quantify the staining lipids.
2.4. Determination of triglyceride and protein levels
Tg accumulation was assessed using a TG color GPO/PAP AA kit (Wiener Laboratory, Rosario, Argentina). Proteins were quantified by the Bradford method using crystalline bovine serum albumin as standard [11].
2.5. MTT assay
To evaluate the toxicity of NAC treatment, MTT (Bromide 3-(4,5-dimethylthiazol-2-yl)-2,5-difeniltetrazol) viability assay was performed [12]. The technique is based on the presence of mitochondrial enzymes in viable cells that reduce the MTT dye and produce a color purple. A stock solution of 5 mg/mL MTT was prepared. To perform the assay, the dye was diluted in complete media to a working concentration of 1 mg/mL. Cells were seeded at 20×104 cells/well and 300 μL of MTT were added to each well. After incubation for 1 h, the medium was removed and the cells were treated with 200 μL of ethanol for 10 min. The resulting solution in each well was transferred to an ELISA plate and its absorbance was measured at 550 nm. To further evaluate toxicity, we performed assays in which cell viability was determined under different concentrations of NAC including 0.01 mM, 0.1 mM, 1 mM and 5 mM. The absorbance of control cells (CC) was set to 100% and the results are presented as percentages of CC.
2.6. Proliferation assay
Cells (50×104) were seeded on a plate and cultured in the presence of vehicle or NAC (0.01 mM, 0.1 mM, 1 mM or 5 mM). The cells were counted at the indicated times. Viable cells were discriminated by trypan blue stain exclusion. Cell number of control cells (CC) was set to 1 and the results are presented as percentages of CC.
2.7. Western blots
Briefly, the experiment was carried out by culturing MEF in medium with 0.5% FBS for one hour to reduce basal levels of phosphorylation; subsequently, different doses of NAC were included in the MDI medium, and the cells were harvested after 30 min of treatment. After the treatment, the cells were lysed with a buffer containing 1% SDS in 60 mM Tris–HCl, boiled for 10 min and, centrifuged at 15,000 rpm at 4 °C for 10 min. Then the samples were resuspended in buffer supplemented with protease inhibitor mixture (Thermo Protease Inhibitor Cocktail). Proteins (40 μg from each sample) were then separated on 10% SDS-polyacrylamide gels and then transferred to nitrocellulose membranes (Amersham; GE Healthcare, Little Chalfont, UK). The primary antibodies were anti-JNK, anti-pJNK, anti-ERK½ and anti-pERK½ (Santa Cruz Biotechnology). The membranes were soaked in blocking buffer (0.1% BSA, 0.4% Tween, and 1 mM EDTA in 0.01 M PBS) for 1 h, incubated overnight with primary antibody at 4 °C. To detect the western blot signal, membranes were probed with horseradish-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, Ca, USA), and then treated with an enhanced chemiluminescence (ECL) substrate kit (Amersham ECL Plus Western Blotting Detection System, GE Healthcare). Results are expressed as arbitrary units.
2.8. Statistics
The results are expressed as the mean±standard deviation (SD). Statistical analysis was performed by one-way analysis of variance followed by post hoc analysis [13]. The results represent the average of four independent experiments (mean±SD). The results were considered statistically significant at p<0.01.
3. Results
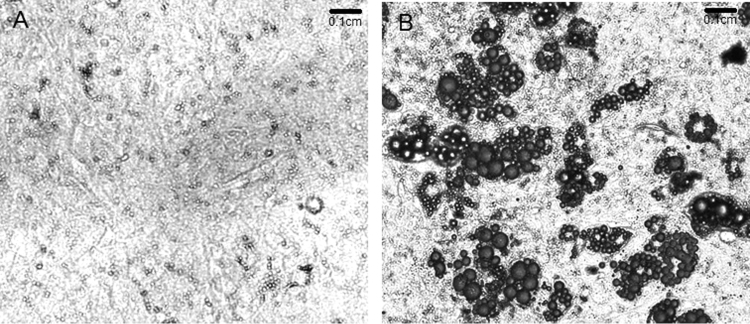
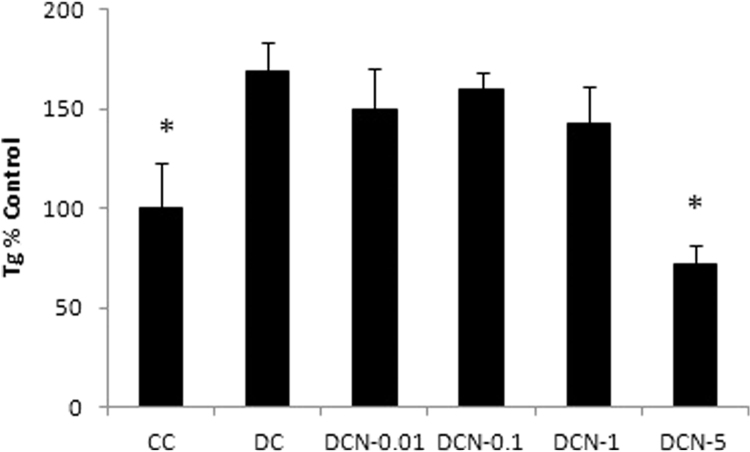
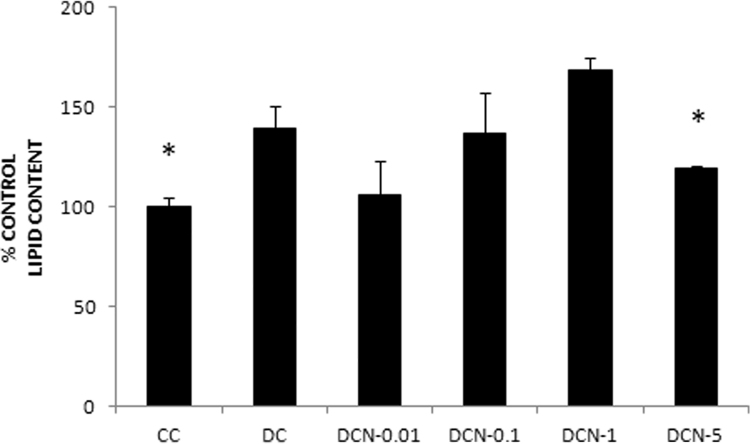
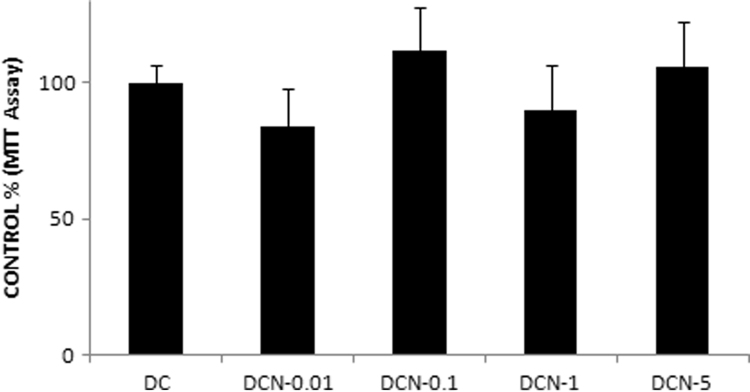
We have previously evaluated the effect of NAC on triglyceride accumulation in 3T3-L1 adipocytes. We demonstrated that 0.01 mM NAC provides effective inhibition of adipocyte differentiation by inhibiting expression of adipogenic transcription factors (PPARγ and C/EBPβ), triglyceride accumulation and ap2 expression [3], [4]. Here, we decided to evaluate the effect of NAC on primary culture MEF, which could differentiate after 10 days (Fig. 1). Fully differentiated cells (DC) showed almost two times more Tg content than did the CC (1.22±0.11 g Tg/g protein [DC] vs. 0.72±0.17 g Tg/g protein [CC], p<0.01). NAC was added to MDI medium at day 0 of the differentiation protocol and replaced every day during all differentiation experiments. Afterwards, we determined the Tg content and, four different NAC doses (0.01 mM, 0.1 mM, 1 mM and 5 mM) were evaluated. Only a significant decrease in Tg accumulation was observed with 5 mM NAC treatment (DCN-5) compared to DC (72±10 [DCN-5] vs 169±15 [DC], p<0.01); DCN-5 showed a Tg content similar to that observed in CC (Fig. 2). We also identified the Oil Red O stained neutral lipids in MEF, allowing us to evaluate both triglyceride and cholesteryl ester contents. Oil Red O stained neutral lipid content was normalized by cell number of each treatment at day 10; it is worth to mention that after day 2 of differentiation protocol the cell number slightly change during the adipogenic differentiation. A significant decrease in DCN-5 was observed (120±2 [DCN-5] vs 139±12 [DC], p<0.01), as shown in Fig. 3, Fig. 4. None of the tested NAC doses provoked toxic effects during cellular differentiation (Fig. 5). Moreover, 5 mM NAC treatment did not produce any effects on control cells (CCN), which showed a Tg content similar to that for CC (0.75±0.06 g Tg/g protein [CCN] vs 0.72±0.17 g Tg/g protein[CC], no significant difference was observed).
Fig. 1.
MEF at day 10 of differentiation. (A) Control cells (CC); (B) MDI-treated cells (DC). Lipid droplets are shown in black. Representative results from one of three experiments with similar results are shown.
Fig. 2.
NAC effect on triglyceride (Tg) accumulation in MEF at day 10 of differentiation. MDI cells were treated with 0.01 mM NAC (DCN-0.01), 0.1 mM NAC (DCN-0.1), 1 mM NAC (DCN-1) or 5 mM NAC (DCN-5). NAC was added at day 0 and replaced every day for 10 days. Control cells (CC), MDI-treated cells (DC) and MDI–NAC-treated cells were harvested at day 10 and, Tg determinations were performed. Cellular Tg content was normalized by cellular protein content. Tg content of control cells (CC) was set to 100, data represent the percentage of CC. The results are the average of four independent experiments (mean±SD). *p<0.01: control cells (CC) vs MDI-treated cells (DC); *p<0.01: MDI – 5 mM NAC-treated cells (DCN-5) vs MDI-treated cells (DC).
Fig. 3.
NAC effect on neutral lipid accumulation visualized with Oil Red O in MEF at day 10 of differentiation. MDI cells were treated with 0.01 mM NAC (DCN-0.01), 0.1 mM NAC (DCN-0.1), 1 mM NAC (DCN-1) or 5 mM NAC (DCN-5). NAC was added at day 0 and replaced every day for 10 days. Control cells (CC), MDI-treated cells (DC) and MDI–NAC-treated cells were harvested at day 10 and Oil Red O dye was extracted and determined. Cellular lipid content was normalized by cell number. Lipid content of control cells (CC) was set to 100, data represent the percentage of CC. The results are the average of four independent experiments (mean±SD). *p<0.01: control cells (CC) vs MDI-treated cells (DC); *p<0.01: MDI – 5 mM NAC treated cells (DCN-5) vs MDI-treated cells (DC).
Fig. 4.
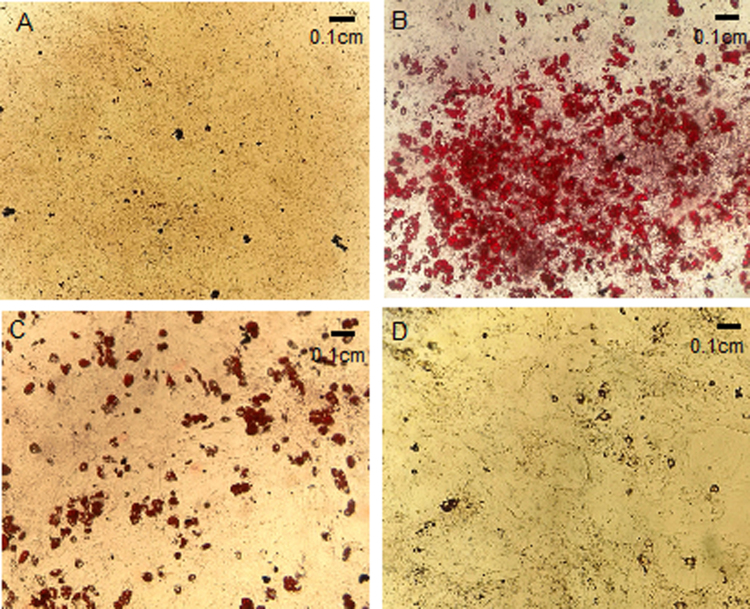
NAC effect on Oil Red O stained neutral lipid in MEF at day 10 of differentiation. Cells were stained with Oil Red O and photographed: (A) Control cells (CC); (B) MDI-treated cells (DC); (C) MDI – 1 mM NAC-treated cells (DCN-1); (D) MDI – 5 mM NAC-treated cells (DCN-5). Stained lipid droplets are shown. Representative results from one of four experiments with similar results are shown.
Fig. 5.
Concentration-dependent effect of NAC on MEF viability. MDI cells were treated with 0.01 mM NAC (DCN-0.01), 0.1 mM NAC (DCN-0.1), 1 mM NAC (DCN-1) or 5 mM NAC (DCN-5). NAC was added at day 0 and replaced every day for 10 days. Control cells (CC), MDI-treated cells (DC) and MDI–NAC-treated cells were harvested at day 10 and, MTT assays were performed. The absorbance of MDI-treated cells (DC) was set to 100% of cell viability, data represent the percentage of CC. The results shown are the averages of four independent experiments (mean±SD).
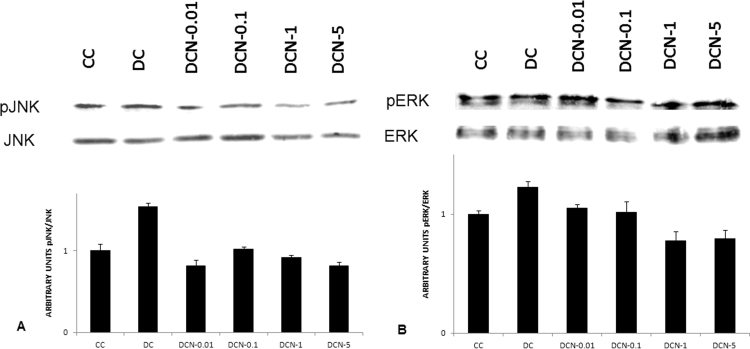
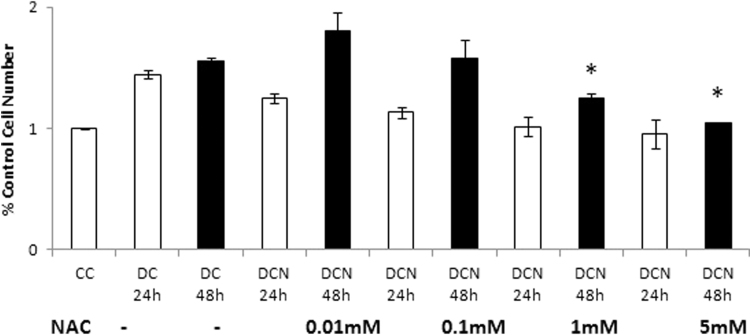
Because MAPK phosphorylation took place in the adipocyte differentiation pathway, we decided to evaluate the effect of NAC on JNK and ERK phosphorylation. Indeed, an increase in expression levels of pJNK and pERK½ in DC were observed. All four doses of NAC (0.01 mM to 5 mM) decreased the levels of pJNK almost 50% (Fig. 6A) without affecting JNK (pJNK/JNK: 0.91±0.05 AU [DCN-5] vs 1.76±0.05 AU [DC]). Likewise, pERK½ expression was slightly inhibited at the highest doses (1 m M and 5 mM) of NAC (Fig. 6(B)). Because ERK½ is involved in cellular proliferation, we evaluated mitotic clonal expansion (MCE) during MEF cellular differentiation. 50% increase in cell number was found in DC compared to CC at 24 h of differentiation protocol, and no significant increase was observed in CC thereafter (48 h: 7.55×105±0.11×105 cells/mL [CC] vs 11.77×105±0.16×105 cells/mL [DC], p<0.01). This observation suggests that a limited mitotic cellular expansion occurred. Only the highest doses of NAC (1 mM and 5 mM) were inhibitory for MCE (48 h: 7.87×105±0.08×105 cells/mL [DCN-5] vs 11.77×105±0.16×105 cells/mL [DC], p<0.01), as shown in Fig. 7.
Fig. 6.
Kinases phosphorylation in response to NAC treatment. (A) pJNK expression was evaluated; the results were normalized to JNK expression. (B) pERK½ expression was evaluated; the results were normalized to ERK½ expression. Comparison of control cells (CC); MDI cells (DC) and MDI + NAC treated cells (DCN-0.01: 0.01 mM NAC; DCN-0.05: 0.05 mM NAC; DCN-1: 1 mM NAC; DCN-5: 5 mM NAC). The results are expressed as arbitrary units, arbitrary units of control cells (CC) was set to 1. The values represent the fold increase in protein expression compared to control cells (CC). The bars show the average of two different experiments (means±SD). Representative results from one of two independent western blot experiments with similar results are shown.
Fig. 7.
NAC effect on MEF during mitotic clonal expansion (MCE). Comparison of control cells (CC); MDI cells (DC); MDI + NAC treated cells (DCN). Cells were harvested at day 1 (DC 24 h, DCN 24 h) or at day 2 (DC 48 h, DCN 48 h) of the differentiation protocol. The concentration of NAC is shown. Cells were counted and viable cells were evaluated by trypan blue stain exclusion. Cell number of control cells (CC) was set to 1, data represent the percentage of CC. Results are the average of four different experiments (mean±SD). *p<0.01: MDI - 1 mM NAC-treated cells vs MDI-treated cells (DC); *p<0.01: MDI – 5 mM NAC-treated cells vs MDI-treated cells (DC).
4. Discusion
The correlation between systemic oxidative stress and fat accumulation, both in humans and in animals, has been shown. However, changes in oxidative metabolism during differentiation from preadipocytes to adipocytes differentiation have not been clearly explained. The mechanism through which antioxidants may cause lipid accumulation inhibition is still controversial [14], [15]. The dose can be critical in regard to inhibiting of inducing preadipocyte differentiation. Understanding adipocyte differentiation is relevant in terms of increasing our knowledge of the pathogenesis of metabolic diseases, as well as in the identification of molecules or pathways that could be suitable target for pharmacological intervention. In this context, we are interested in the role of glutathione precursors, such as the NAC. Wang et al. [16] demonstrated that mice that consumed NAC decreased their body fat, whereas Gou and collaborators [17] suggested that beta-mercapto-ethanol (also precursor of glutathione as NAC), would increase expression of adipogenic genetic markers and accumulation of lipids. Due to the controversial reports regarding the effect of glutathione precursors as antioxidants on lipid accumulation, their possible use in this condition is still an open issue. In this way, we decided to evaluate JNK signaling pathway, which is activated by oxidative cellular stress [18] and phosphorylated during adipogenic differentiation [19], [20]. We observed an increase in expression levels of pJNK in DC; NAC treatments produced an effective inhibition of pJNK expression, independent of NAC doses. We did not evaluate ROS in MEF, but we suggest that antioxidant environment could be responsible for decrease expression of pJNK.
In our laboratory we have studied some points that have not yet been clarified, such as GSH metabolism and the effect of antioxidants on the adipogenic differentiation of the 3T3-L1 preadipocytes. In our experience, NAC could inhibit triglyceride accumulation during 3T3-L1 adipogenic differentiation, concomitant with changes in GSH cellular content and through inhibition of adipogenic transcription factor expression [3]. As a consequence, we could temporarily relate the parameters of oxidative stress with triglyceride production. We have also shown that NAC inhibited the expression of some adipocyte proteins expression such as FABP4 (ap2), by almost 90% [4]. In this context, we decided to evaluate the effect of NAC on MEF primary cultures. We showed for the first time that the highest dose of NAC effectively inhibited triglyceride accumulation in NAC-treated MEF adipocytes, but the antioxidant slightly inhibited Oil Red O neutral lipid accumulation in the cells. Meanwhile, none of the doses produced toxic effect on the cells.
Kinetic studies showed that NAC decreased the expression of PPARγ by affecting CEBP/β expression without altering the phosphorylation of AKT [3]. Here, we observed that the highest doses of NAC could decrease pERK½ expression, as well as inhibit MCE in MEF cells during adipogenic differentiation. These results suggest the effect of NAC on lipid accumulation may be mediated, at least in part, by affecting the activation of this kinase, as part of the MAPK pathway.
Acknowledgements
This study was supported by Grant UBACYT 20020130200160BA (Universidad de Buenos Aires, Argentina), OAT No. 42/2013 - 2015 (Universidad de Buenos Aires, Argentina). Juan Carlos Calvo, Ma del Carmen Vila and Liliana N. Guerra are researchers funded by CONICET (Ministerio de Ciencia y Técnica, Argentina) and Universidad de Buenos Aires.
References
- 1.Liu G.S., Chan E.C., Higuchi M., Dusting G.J., Jiang F. Redox mechanisms in regulation of adipocyte differentiation: beyond a general stress response. Cells. 2012;1:976–993. doi: 10.3390/cells1040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calzadilla P., Gomez-Serrano M., García-Santos E., Schiappacasse A., Abalde Y., Calvo J.C., Peral B., Guerra L.N. N- Acetylcysteine affects obesity proteins expression in 3T3-L1. Redox Rep. 2013;18:210–218. doi: 10.1179/1351000213Y.0000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calzadilla P., Sapochnik D., Cosentino S., Diz V., Dicelio L., Calvo J.C., Guerra L.N. N-acetylcysteine reduces markers of differentiation in 3T3-L1 adipocytes. Int. J. Mol. Sci. 2011;12:6936–6951. doi: 10.3390/ijms12106936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prusty D., Park B.H., Davis K.E., Farmer S.R. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma) and C/EBP alpha gene expression during differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 2002;27:46226–46232. doi: 10.1074/jbc.M207776200. [DOI] [PubMed] [Google Scholar]
- 5.Donzelli E., Lucchini C., Ballarini E., Scuteri A., Carini F., Tredici G., Miloso M. ERK1 and ERK 2 are involved in recruitment and maturation of human mesenchymal stem cells induced to adipogenic differentiation. J. Mol. Cell Biol. 2011;3:123–131. doi: 10.1093/jmcb/mjq050. [DOI] [PubMed] [Google Scholar]
- 6.Bost F., Aouadi M., Caron L., Binetruy B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie. 2005;87:51–56. doi: 10.1016/j.biochi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Gehart H., Kumpf S., Ittner A., Ricci R. MAPK signaling in cellular metabolism: stress or wellness? EMBO Rep. 2010;11:834–840. doi: 10.1038/embor.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J.R., Ryu H., Chung H.J., Lee J.H., Kim S.W., Kwun W.H., Baek S.H., Kim J.H. Association of anti-obesity activity of N-acteylcysteine with metalothionein-II down-regulation. Experimental. Mol. Med. 2006;30:162–172. doi: 10.1038/emm.2006.20. [DOI] [PubMed] [Google Scholar]
- 9.Araki S., Dobashi K., Kugo K., Yamamoto Y., Asayama K., Shirahata A. N-acetylcysteine attenuates TNF-alpha induced changes in secretion of interleukin-6, plaminogen activator inhibitor-1 and adiponectin from 3T3-L1 adipocytes. Life Sci. 2006;79:2405–2412. doi: 10.1016/j.lfs.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Lukas J., Bartkova J., Rohde M., Strauss M., Bartek J. Cyclin D1 is dispensable for G1 control in retinoblastoma gene-deficient cells independently of cdk4 activity. Mol. Cell Biol. 1995;15:2600–2611. doi: 10.1128/mcb.15.5.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 12.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 13.Analytical Software . Mcgraw–Hill/Irwin; Tallahassee, FL, USA: 2003. Statistix 8. [Google Scholar]
- 14.Ristow M., Zarse K., Oberbach A., Kloting N., Brirringer M., Kiehntopt M., Stumvoll M., Kahn R., Bluher M. Antioxidants prevents health promoting effects of physical exercise in humans. Proc. Nat. Acad. Sciences USA. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uto – Kondo H., Ohmori R., Kiyose C., Kishimoto Y., Saito H., Igarashi O., Kondo K. Tocotrienol suppresses adipocyte differentiation and AKT phosphorylation in 3T3-L1 preadipocytes. J. Nutr. 2009;139:51–57. doi: 10.3945/jn.108.096131. [DOI] [PubMed] [Google Scholar]
- 16.Wang T., Si Y., Shirihai O.S., Si H., Schultz V., Corkey R.F. Respiration in adipocytes is inhibited by reactive oxygen species. Obesity. 2010;18:1493–1502. doi: 10.1038/oby.2009.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo W., Li Y., Liang W., Wong S., Apovian C., Kirkland B. Beta-mercaptoethanol suppresses inflammation and induces adipogenic differentiation in 3T3-F442 murine preadipocytes. PLoS One. 2012;7:e40958. doi: 10.1371/journal.pone.0040958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim E.K., Choi E.J. Compromised MAPK signaling in human diseases: an update. Arch. Toxicol. 2015;89:867–882. doi: 10.1007/s00204-015-1472-2. [DOI] [PubMed] [Google Scholar]
- 19.Poudel B., Lim S., Ki H., Nepali S., Lee Y., Kim D. Dioscin inhibits adipogenesis through the AMPK/MAPK pathway in 3T3-L1 and modulates fat accumulation in obese mice. Int. J. Mol. Med. 2014;34:1401–1408. doi: 10.3892/ijmm.2014.1921. [DOI] [PubMed] [Google Scholar]
- 20.Li K.K., Liu C.L., Shiu H.T., Wong H.L., Siu W.S., Zhan C. Cocoa tea (Camellia ptilophylla) water extract inhibits adipocyte differentiation in mouse 3T3-L1 preadipocytes. Sci. Rep. 2016;6:20172–20183. doi: 10.1038/srep20172. [DOI] [PMC free article] [PubMed] [Google Scholar]