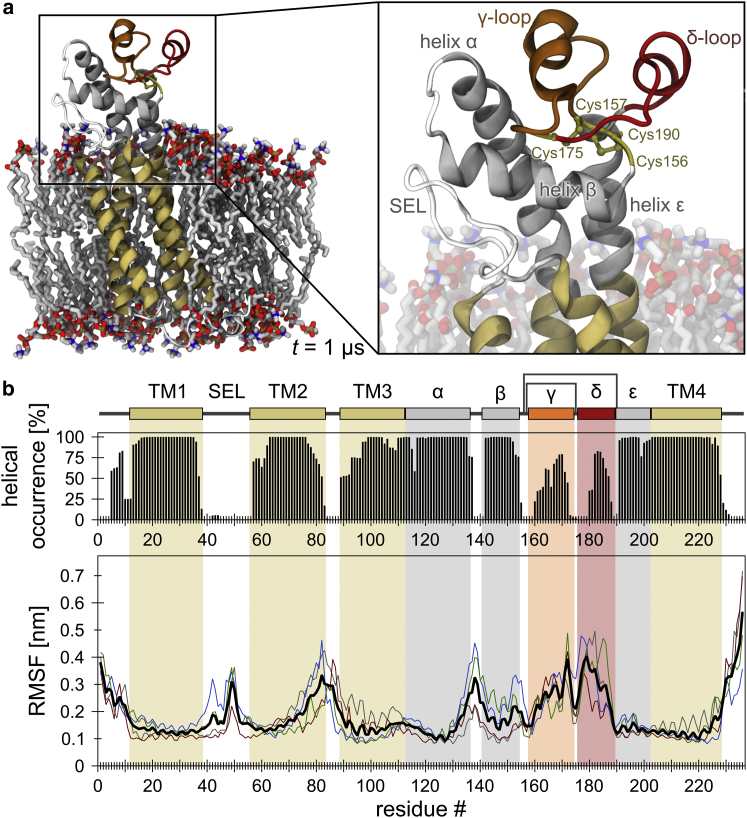
Figure 2.
Helical stability and backbone flexibility of CD81FL. (a) Left: conformation of the full-length protein embedded in a POPC bilayer after a 1 μs unbiased, atomistic MD simulation (run D; see also Fig. S2b). TM1–4 in dark yellow; helices α, β, and ε in gray; loops γ and δ in orange and red, respectively. Red, blue, and tan atoms of the lipid bilayer represent oxygen, nitrogen, and phosphate, respectively. Right: enlarged view of the SEL and LEL. (b) Helical structure per residue (top) and Cα RMSF (bottom). Note that a direct comparison of the average RMSFs in (b) (shown in black; n = 4) and Fig. 1b suggests that the LEL domain flexibility increases in CD81FL; however, this is a result of least-square fitting to all Cα atoms of the full-length protein (for comparison of exclusively LEL segments, see Fig. 3a). To see this figure in color, go online.