Abstract
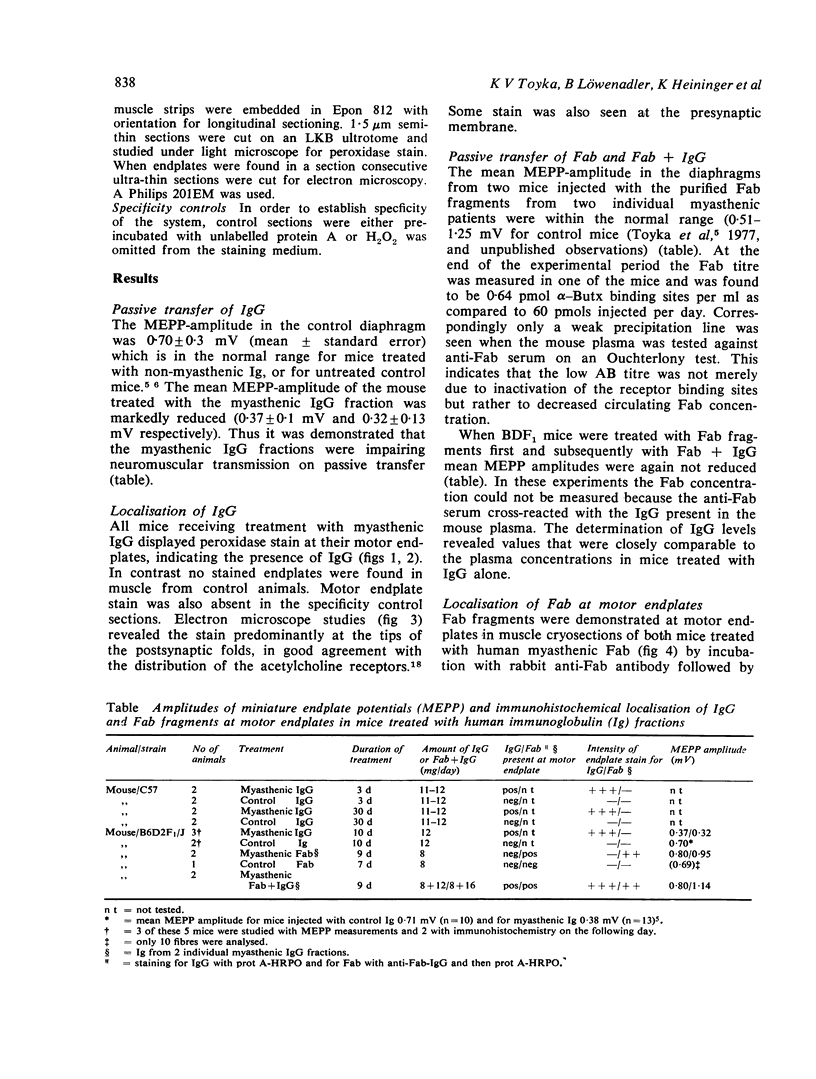
Using the mouse passive transfer model, the effects of purified human myasthenic immunoglobulin G and of the monovalent Fab fragment on neuromuscular junctions were investigated. Treatment with IgG markedly reduced amplitudes of miniature endplate potentials. When Fab fragments were transferred alone or with subsequent addition of IgG no neuromuscular transmission block was induced. Myasthentic IgG and Fab were specifically demonstrated at the neuromuscular junctions by immunohistochemistry. On electronmicroscopy endplate structure was normal in transfer experiments using IgG for up to 30 days. It is suggested that Fab fragments bind to acetylcholine receptors without affecting transmission and protect them from the attack of complete IgG antibodies.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Rash J. E., Mayer R. F., Satterfield J. R. An electrophysiological and morphological study of the neuromuscular junction in patients with myasthenia gravis. Exp Neurol. 1976 Jun;51(3):536–563. doi: 10.1016/0014-4886(76)90179-5. [DOI] [PubMed] [Google Scholar]
- Appel S. H., Anwyl R., McAdams M. W., Elias S. Accelerated degradation of acetylcholine receptor from cultured rat myotubes with myasthenia gravis sera and globulins. Proc Natl Acad Sci U S A. 1977 May;74(5):2130–2134. doi: 10.1073/pnas.74.5.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend W. P., Silverblatt F. J. Serum disappearance and catabolism of homologous immunoglobulin fragments in rats. Clin Exp Immunol. 1975 Dec;22(3):502–513. [PMC free article] [PubMed] [Google Scholar]
- Drachman D. B., Angus C. W., Adams R. N., Michelson J. D., Hoffman G. J. Myasthenic antibodies cross-link acetylcholine receptors to accelerate degradation. N Engl J Med. 1978 May 18;298(20):1116–1122. doi: 10.1056/NEJM197805182982004. [DOI] [PubMed] [Google Scholar]
- Drachman D. B. Myasthenia gravis (first of two parts). N Engl J Med. 1978 Jan 19;298(3):136–142. doi: 10.1056/NEJM197801192980305. [DOI] [PubMed] [Google Scholar]
- Engel A. G., Lambert E. H., Howard F. M. Immune complexes (IgG and C3) at the motor end-plate in myasthenia gravis: ultrastructural and light microscopic localization and electrophysiologic correlations. Mayo Clin Proc. 1977 May;52(5):267–280. [PubMed] [Google Scholar]
- Engel A. G., Santa T. Histometric analysis of the ultrastructure of the neuromuscular junction in myasthenia gravis and in the myasthenic syndrome. Ann N Y Acad Sci. 1971 Sep 15;183:46–63. doi: 10.1111/j.1749-6632.1971.tb30741.x. [DOI] [PubMed] [Google Scholar]
- Fertuck H. C., Salpeter M. M. Localization of acetylcholine receptor by 125I-labeled alpha-bungarotoxin binding at mouse motor endplates. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1376–1378. doi: 10.1073/pnas.71.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn E., Mattsson C., Thornell L. E., Sjöström M., Stålberg E., Hilton-Brown P., Elmqvist D. Experimental myasthenia in rabbits: biochemical, immunological, electrophysiological, and morphological aspects. Ann N Y Acad Sci. 1976;274:337–353. doi: 10.1111/j.1749-6632.1976.tb47696.x. [DOI] [PubMed] [Google Scholar]
- Heilbronn E., Stålberg E. The pathogenesis of myasthenia gravis. J Neurochem. 1978 Jul;31(1):5–11. doi: 10.1111/j.1471-4159.1978.tb12426.x. [DOI] [PubMed] [Google Scholar]
- Kao I., Drachman D. B. Myasthenic immunoglobulin accelerates acetylcholine receptor degradation. Science. 1977 Apr 29;196(4289):527–529. doi: 10.1126/science.850793. [DOI] [PubMed] [Google Scholar]
- Karnovsky M. J. The ultrastructural basis of capillary permeability studied with peroxidase as a tracer. J Cell Biol. 1967 Oct;35(1):213–236. doi: 10.1083/jcb.35.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert E. H., Lindstrom J. M., Lennon V. A. End-plate potentials in experimental autoimmune myasthenia gravis in rats. Ann N Y Acad Sci. 1976;274:300–318. doi: 10.1111/j.1749-6632.1976.tb47694.x. [DOI] [PubMed] [Google Scholar]
- Lefvert A. K., Bergström K., Matell G., Osterman P. O., Pirskanen R. Determination of acetylcholine receptor antibody in myasthenia gravis: clinical usefulness and pathogenetic implications. J Neurol Neurosurg Psychiatry. 1978 May;41(5):394–403. doi: 10.1136/jnnp.41.5.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon V. A., Seybold M. E., Lindstrom J. M., Cochrane C., Ulevitch R. Role of complement in the pathogenesis of experimental autoimmune myasthenia gravis. J Exp Med. 1978 Apr 1;147(4):973–983. doi: 10.1084/jem.147.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon V. A. The immunopathology of myasthenia gravis. Hum Pathol. 1978 Sep;9(5):541–551. doi: 10.1016/s0046-8177(78)80135-x. [DOI] [PubMed] [Google Scholar]
- Mihovilovic M., Martinez-Carrion M. Purification of high-affinity Fab fragments from experimental autoimmune myasthenia gravis rabbits and their effect on isolated acetylcholine receptors. Biochemistry. 1979 Oct 16;18(21):4522–4528. doi: 10.1021/bi00588a010. [DOI] [PubMed] [Google Scholar]
- NAGEL W., WILLIG F., PESCHKE W., SCHMIDT F. H. UBER DIE BESTIMMUNG VON TRYPSIN UND CHYMOTRYPSIN MIT AMINOSAEURE-P-NITROANILIDEN. Hoppe Seylers Z Physiol Chem. 1965;340:1–10. doi: 10.1515/bchm2.1965.340.1-2.1. [DOI] [PubMed] [Google Scholar]
- Ruddy S., Gigli I., Austen K. F. The complement system of man. I. N Engl J Med. 1972 Sep 7;287(10):489–495. doi: 10.1056/NEJM197209072871005. [DOI] [PubMed] [Google Scholar]
- Smith T. W., Lloyd B. L., Spicer N., Haber E. Immunogenicity and kinetics of distribution and elimination of sheep digoxin-specific IgG and Fab fragments in the rabbit and baboon. Clin Exp Immunol. 1979 Jun;36(3):384–396. [PMC free article] [PubMed] [Google Scholar]
- Stanley E. F., Drachman D. B. Effect of myasthenic immunoglobulin on acetylcholine receptors of intact mammalian neuromuscular junctions. Science. 1978 Jun 16;200(4347):1285–1287. doi: 10.1126/science.663610. [DOI] [PubMed] [Google Scholar]
- Toyka K. V., Birnberger K. L., Anzil A. P., Schlegel C., Besinger U., Struppler A. Myasthenia gravis: further electrophysiological and ultrastructural analysis of transmission failure in the mouse passive transfer model. J Neurol Neurosurg Psychiatry. 1978 Aug;41(8):746–753. doi: 10.1136/jnnp.41.8.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyka K. V., Brachman D. B., Pestronk A., Kao I. Myasthenia gravis: passive transfer from man to mouse. Science. 1975 Oct 24;190(4212):397–399. doi: 10.1126/science.1179220. [DOI] [PubMed] [Google Scholar]
- Toyka K. V., Drachman D. B., Griffin D. E., Pestronk A., Winkelstein J. A., Fishbeck K. H., Kao I. Myasthenia gravis. Study of humoral immune mechanisms by passive transfer to mice. N Engl J Med. 1977 Jan 20;296(3):125–131. doi: 10.1056/NEJM197701202960301. [DOI] [PubMed] [Google Scholar]






