Abstract
The present study aimed to investigate the clinical significance of cancer embryo antigen (CEA) and endothelin-1 (ET-1) in the exhaled breath condensate (EBC) of patients with non-small cell lung cancer (NSCLC). EBC samples were collected from 143 patients with NSCLC and 119 healthy individuals by using an EBC collector. The CEA and ET-1 levels in the EBC and serum were detected. The levels of CEA and ET-1 in the serum and EBC of the NSCLC group were higher compared with those of the healthy group. The level of CEA in the EBC of the adenocarcinoma group was higher compared with that in the squamous cell carcinoma group. The levels of CEA and ET-1 in the serum and EBC in stages III and IV were higher compared with those in stages I and II. The levels of CEA and ET-1 in the EBC were positively correlated with those in the serum, and furthermore, they exhibited high specificity and sensitivity. Thus, these parameters may be used to diagnose lung cancer. The detection of CEA and ET-1 in EBC may help the process of diagnosing and monitoring the progression of NSCLC.
Keywords: non-small cell lung cancer, exhaled breath condensate, cancer embryo antigen, endothelin-1
Background
Lung cancer is considered to be one of the leading causes of mortality worldwide in the 21st century. Millions of individuals die from lung cancer annually (1). Mortality caused by lung cancer ranks first among other cancer types, regardless of gender. Among lung cancer cases, non-small cell lung cancer (NSCLC) accounts for 80–85% of the total. Although medical treatments for NSCLC have greatly progressed, this cancer type continues to be associated with one of the most malignant types of tumour, with the worst post-operative therapeutic effects (2). With the help of early diagnosis and treatment, the five-year survival rate of patients with NSCLC has increased (3). Therefore, researchers have aimed to develop novel reliable and atraumatic methods to diagnose and assess lung cancer. Similarly, the present study aimed to develop a novel method that may be used to diagnose and evaluate lung cancer.
As an indication of airway canceration, exhaled breath condensate (EBC) comprises the liquid and volatile mixture secreted by the lower respiratory tract mucosa (4). EBC collection techniques exhibit several advantages, including non-invasiveness, simplicity in their execution and high repeatability (5,6). The detection of tumour markers in EBC may be a novel approach to diagnose lung cancer in the early stages and in screens of high-risk groups (7–10). In the present study, cancer embryo antigen (CEA) and endothelin-1 (ET-1) were detected in the EBC of 133 patients with NSCLC.
Subjects and methods
Study subjects
A total of 143 patients were diagnosed with squamous cell carcinoma and adenocarcinoma, who received bronchoscopy, lung biopsy and open chest surgery in our hospital from February 2011 to October 2014. The patients with severe heart disease and damaged liver or kidney functions, and those who failed to cooperate during the study, were excluded. Of the 143 subjects, 75 suffered from adenocarcinoma and 68 endured squamous cell carcinoma. According to the seventh version of the lung cancer tumour-node-metastasis staging standard provided by the Union for International Cancer Control in 2009 (11), 15 cases were in stage I, 29 cases were in stage II, 59 cases were in stage III and 40 cases were in stage IV. Moreover, 119 healthy volunteers were included in the normal control group. No statistically significant differences were identified in terms of the age, gender or smoking history of the participants.
Sample collection
EBC was collected using a HAAK EK20 EcoScreen (Eric Jaeger, Friedberg, Germany). The subjects wore nose-clips and maintained eupnoea for 20 min by biting mouthparts. This method was used to collect 1–3 ml condensate from each subject; the obtained condensate was subsequently preserved at −70°C. Simultaneously, 3 ml fasting venous blood was drawn early in the morning, and the serum was extracted. The samples were allowed to coagulate at room temperature for 30 min, and subsequently were centrifuged at 2,500 g for 20 min. The samples were preserved at −20°C, and the test was conducted within a week.
Testing method
Chemiluminescence microparticle immunoassays were performed using a CEA kit (Abbott Laboratories, Abbott Park, IL, USA). An enzyme-linked immunosorbent assay (ELISA) was performed using an ET-1 kit (R&D Systems, Inc., Minneapolis, MN, USA). The experiment was performed in accordance with the manufacturer's protocol.
Statistical analysis
Data were statistically analysed using SPSS 13.0 software (SPSS, Inc., Chicago,. IL, USA). Measured data were in accordance with the normal distribution of the mean ± standard deviation (x̅ ± s) under a normal distribution test. The two samples were compared using a t-test, and the measured data were subjected to a χ2 test. The correlation between CEA and ET-1 in the EBC and serum was determined through correlational analysis. Receiver operating characteristic (‘ROC’) curve analysis was performed to determine the specificity and sensitivity of the method to diagnose lung cancer. P<0.05 was considered to indicate a statistically significant value.
Results
The levels of CEA and ET-1 in the serum and EBC samples were compared between the NSCLC group and the healthy group (Table I), revealing that the CEA levels in the EBC and serum of the lung cancer group were significantly higher compared with those of the normal control group (P<0.01). The levels of CEA and ET-1 in the serum and EBC samples were also compared between the two pathological lung cancer types investigated (adenocarcinoma and squamous cell carcinoma) (Table II). This analysis determined that the level of CEA in the EBC and serum of the patients with adenocarcinoma was significantly higher compared with that of the patients with squamous cell carcinoma.
Table I.
Comparison of the CEA and ET-1 levels in the serum and EBC of the two groups (x̅ ± s).
| EBC | Serum | ||||
|---|---|---|---|---|---|
| Parameter | N | CEA (mg/l) | ET-1 (ng/l) | CEA (mg/l) | ET-1 (ng/l) |
| Subject | |||||
| NSCLC | 143 | 2.96±1.54 | 19.68±7.41 | 14.52±7.48 | 63.30±20.04 |
| Healthy | 119 | 0.82±0.42 | 7.35±3.15 | 3.59±2.07 | 46.51±15.16 |
| t-test | 14.753 | 16.913 | 15.455 | 7.520 | |
| P-value | <0.01 | <0.01 | <0.01 | <0.01 | |
CEA, cancer embryo antigen; ET-1, endothelin-1; EBC, exhaled breath condensate; NSCLC, non-small-cell lung cancer.
Table II.
Association between the levels of CEA and ET-1 and the pathological type (x̅ ± s).
| EBC | Serum | ||||
|---|---|---|---|---|---|
| Parameter | N | CEA (mg/l) | ET-1 (ng/l) | CEA (mg/l) | ET-1 (ng/l) |
| Cancer type | |||||
| Adenocarcinoma | 75 | 3.99±1.09 | 20.07±7.24 | 19.27±5.87 | 64.96±21.87 |
| Squamous cell | 68 | 1.82±1.08 | 19.25±7.63 | 9.28±5.23 | 61.46±17.80 |
| carcinoma | |||||
| t-test | 11.946 | 0.658 | 3.977 | 1.044 | |
| P-value | <0.01 | >0.05 | <0.01 | >0.05 | |
CEA, cancer embryo antigen; ET-1, endothelin-1; EBC, exhaled breath condensate.
The correlation of the levels of CEA and ET-1 with lung cancer staging was subsequently determined (Table III). These results revealed that the levels of CEA in the EBC and serum of the patients with lung cancer stages III and IV were significantly higher compared with those of the patients with lung cancer stage I and II (P<0.01).
Table III.
Relationship of the CEA and ET-1 levels with lung cancer staging (x̅ ± s).
| EBC | Serum | ||||
|---|---|---|---|---|---|
| Parameter | N | CEA (mg/l) | ET-1 (ng/l) | CEA (mg/l) | ET-1 (ng/l) |
| Stage I/IIa | 44 | 1.53±1.18 | 15.29±8.22 | 8.48±5.29 | 53.73±21.10 |
| Stage III/IV | 99 | 3.59±1.22 | 21.63±6.12 | 17.20±6.72 | 67.55±18.09 |
| t-test | 9.410 | 5.125 | 7.623 | 4.000 | |
| P-value | <0.01 | <0.01 | <0.01 | <0.01 | |
The cancer stages (I–IV) were assigned according to the seventh version of the lung cancer tumour-node-metastasis staging standard provided by the Union for International Cancer Control in 2009 (11). CEA, cancer embryo antigen; ET-1, endothelin-1; EBC, exhaled breath condensate.
The correlation between the levels of CEA and ET-1 in the EBC and serum is shown in Figs. 1 and 2. These results demonstrated a positive linear correlation between the levels of CEA identified in the EBC and in serum CEA (Fig. 1); a similar result was identified with the level of ET-1 in the EBC and the serum: The correlation coefficients (r) were calculated to be 0.884 and 0.675, respectively (P<0.05).
Figure 1.
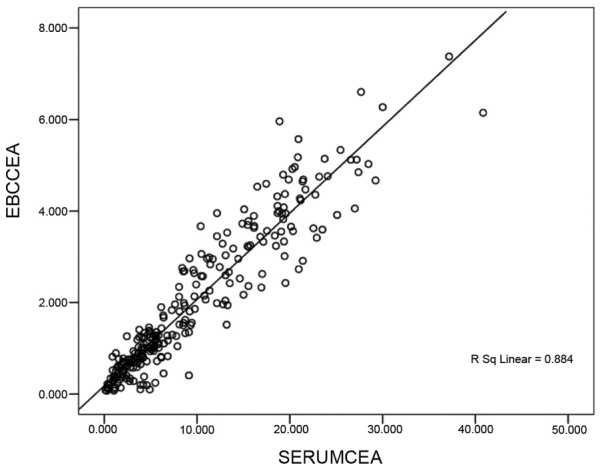
Correlation scatter-diagram of CEA in the EBC and serum. CEA, cancer embryo antigen; EBC, exhaled breath condensate.
Figure 2.
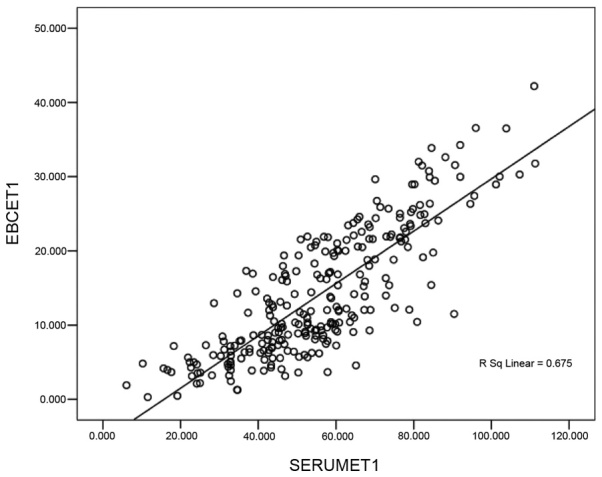
Correlation scatter diagram of ET-1 in the EBC and serum. CEA, cancer embryo antigen; EBC, exhaled breath condensate.
Subsequently, the sensitivity and specificity of the levels of CEA and ET-1 were analysed to diagnose lung cancer (Fig. 3 and Table IV).
Figure 3.
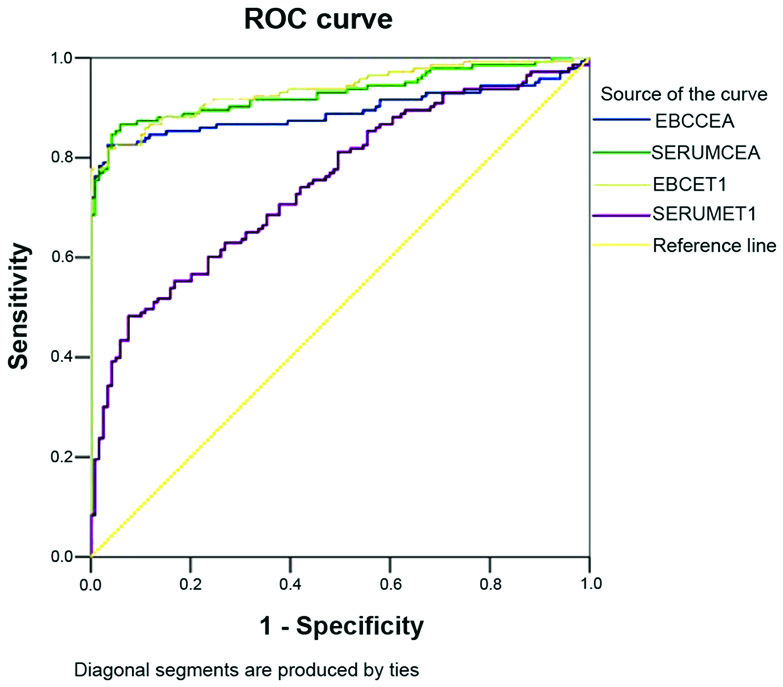
ROC curve of the levels of CEA and ET-1 in the EBC and serum. The measurement data are derived from Figs. 1 and 2. ROC, receiver operating characteristic; CEA, cancer embryo antigen; EBC, exhaled breath condensate.
Table IV.
Specificity and sensitivity of CEA and ET-1 in the EBC and serum to diagnose lung cancer.
| Parameter | Area under the ROC curve | Critical value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| EBC CEA | 0.893 | 1.505 mg/l | 82.5 | 96.6 |
| Serum CEA | 0.929 | 7.063 mg/l | 84.6 | 95.8 |
| EBC ET-1 | 0.936 | 12.681 ng/l | 81.8 | 96.6 |
| Serum ET-1 | 0.748 | 65.334 ng/l | 48.0 | 92.4 |
CEA, cancer embryo antigen; ET-1, endothelin-1; EBC, exhaled breath condensate; ROC, receiver operating characteristic.
Discussion
EBC provides a novel tool to detect the biochemical constituents of the respiratory tract; EBC does not interfere with the physiological or pathological processes of the respiratory tract (12). Collection of the EBC is completely atraumatic, and does not harm the bronchial mucosa. Furthermore, EBC is directly collected from the airways, and dilution is impossible. Therefore, the results from such a study may be considered to be reliable and highly repeatable (13). Thus far, studies have focused on markers of inflammation in the EBC, including nitric oxide, carbon monoxide, 8-iso-prostaglandin and leukotriene (14–16). However, researchers have rarely investigated EBC tumour markers (17,18). Figs. 1 and 2 revealed a positive linear correlation between the levels of CEA determined in the EBC and in the serum. A similar correlation was also observed between the levels of ET-1 in the EBC and the serum. The correlation coefficients were 0.884 and 0.675, respectively (P<0.05). These results indicated that this reliable and atraumatic EBC collection method may be used to effectively detect the levels of CEA and ET-1 in patients. In addition, this method also helped in the auxiliary diagnosis of NSCLC.
CEA is one of the tumour markers used to diagnose NSCLC. Table I indicated that the levels of CEA in the EBC and serum of the lung cancer group were significantly higher compared with those of the normal control group (P<0.01); this result is consistent with the findings of a previous study (19). In addition, the CEA and ET-1 levels in the EBC exhibited sensitivity (82.5% for EBC CEA; 81.8% for EBC ET-1) and specificity (96.6% for both EBC CEA and EBC ET-1) to diagnose lung cancer; thus, the level of CEA in the EBC may help in the auxiliary diagnosis of NSCLC. EBC CEA serves as a supplementary diagnostic index for lung cancers that cannot be specifically diagnosed through traumatic detection, for example, via bronchoscopy.
CEA is considered to be the preferred indicator of lung adenocarcinoma (20). Table II shows that the positive expression rates of CEA in squamous cell carcinoma and adenocarcinoma were 42.9 and 73.3%, respectively. The level of CEA in the EBC and serum of adenocarcinoma patients was higher compared with that of squamous cell carcinoma patients: This finding is consistent with those described in other studies (19,21). This finding also revealed that the level of CEA in the EBC is valuable for the diagnosis of pathological lung cancer types. This analysis indicated that the levels of CEA in the EBC and serum of patients with lung cancer in stages III and IV were higher compared with those of patients with lung cancer in stages I and II (Table III). The level of CEA is likely to increase as the disease progresses (21), and this pattern revealed that the regular monitoring of EBC CEA may contribute to the assessment of lung cancer development. Regular monitoring may also serve as a guide for clinical treatments (22).
ET-1 is an active peptide of 21 amino acid residues from vascular endothelial cells. ET-1 is also a strong accelerant of cell mitosis. ET-1 is able to promote tumour cell proliferation and capillary formation (23). The highest content of ET-1 is to be found in the kidney, followed by the lungs; thus, the cells in these organs are conducive to DNA synthesis and proliferation. Previous studies have revealed that an increase in the levels of endothelin-converting enzyme results in an increase in ET-1 secretory volume. The final process of the endothelin-converting enzyme, which catalyses endothelin synthesis, is closely associated with the biological behaviour of several malignant tumours. However, only a few studies have focused on ET-1 in EBC. An ELISA was performed in the present study to detect ET-1 in the EBC and serum. This method confirmed that the levels of ET-1 in the EBC and serum of the NSCLC group were higher compared with those in the normal control group (Table I). The specificity and sensitivity of the method to detect ET-1 in the EBC were higher compared with those to detect ET-1 in the serum; therefore, detection of ET-1 in the EBC is of greater importance for the auxiliary diagnosis of NSCLC with respect to the detection of ET-1 in the serum.
Table III also revealed that the ET-1 levels in the EBC and serum of the patients with lung cancer in stages III and IV were higher compared with those of patients with lung cancer in stages I and II. The level of ET-1 increased as the disease progresses; this finding is consistent with that of Carpagnano et al (24) and Boldrini et al (25). This tendency indicated that ET-1 induces the growth and metastasis of lung cancer. The presence of a high level of ET-1 would correspond to a high likelihood of metastasis and the worst post-operative effects. ET-1 in the EBC may be used as an index to monitor lung cancer metastasis and to assess the effect of surgery. This is a possibility, partly due to the fact that ET-1 is a hypoxia-inducible angiogenic growth factor. ET-1 promotes angiogenesis in lung cancer, DNA synthesis and cell proliferation; thus, ET-1 contributes to the growth and metastasis of lung tumours.
In conclusion, CEA and ET-1 may be detected in the EBC of patients with NSCLC, and these parameters are of great importance in the early diagnosis, monitoring, pathological type determination and prognosis of NSCLC. As a novel approach to investigate patients with NSCLC, the collection of EBC is easy, atraumatic, repeatable and comparable. Therefore, this technique should be further promoted.
Acknowledgements
The present study was supported by a Clinical Key Speciality Project of China.
References
- 1.Eberini I, Gianazza E, Pastorino U, Sirtori C. Assessment of individual lung cancer risk by the proteomic analysis of exhaled breath condensate. Expert Opin Med Diagn. 2008;2:1309–1315. doi: 10.1517/17530050802600675. [DOI] [PubMed] [Google Scholar]
- 2.Pillai RN, Ramalingam SS. Advances in the diagnosis and treatment of non-small cell lung cancer. Mol Cancer Ther. 2014;13:557–564. doi: 10.1158/1535-7163.MCT-13-0669. [DOI] [PubMed] [Google Scholar]
- 3.Padda SK, Burt BM, Trakul N, Wakelee HA. Early-stage non-small cell lung cancer: Surgery, stereotactic radiosurgery and individualized adjuvant therapy. Semin Oncol. 2014;41:40–56. doi: 10.1053/j.seminoncol.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Dalaveris E, Kerenidi T, Katsabeki-Katsafli A, Kiropoulos T, Tanou K, Gourgoulianis KI, Kostikas K. VEGF, TNF-alpha and 8-isoprostane levels in exhaled breath condensate and serum of patients with lung cancer. Lung Cancer. 2009;64:219–225. doi: 10.1016/j.lungcan.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Ciebiada M, Górski P, Antczak A. Eicosanoids in exhaled breath condensate and bronchoalveolar lavage fluid of patients with primary lung cancer. Dis Markers. 2012;32:329–335. doi: 10.1155/2012/562862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antus B, Barta I. Exhaled breath condensate pH in patients with lung cancer. Lung Cancer. 2012;75:178–180. doi: 10.1016/j.lungcan.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Chan HP, Lewis C, Thomas PS. Exhaled breath analysis: Novel approach for early detection of lung cancer. Lung Cancer. 2009;63:164–168. doi: 10.1016/j.lungcan.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 8.Mozzoni P, Banda I, Goldoni M, Corradi M, Tiseo M, Acampa O, Balestra V, Ampollini L, Casalini A, Carbognani P, Mutti A. Plasma and EBC microRNAs as early biomarkers of non-small-cell lung cancer. Biomarkers. 2013;18:679–686. doi: 10.3109/1354750X.2013.845610. [DOI] [PubMed] [Google Scholar]
- 9.Stathopoulos D, Loukides S, Syrigos K. 8-Isoprostane in exhaled breath condensate of patients with non-small cell lung cancer: The effect of chemotherapy. Anticancer Res. 2014;34:5143–5145. [PubMed] [Google Scholar]
- 10.Brussino L, Culla B, Bucca C, Giobbe R, Boita M, Isaia G, Heffler E, Oliaro A, Filosso P, Rolla G. Inflammatory cytokines and VEGF measured in exhaled breath condensate are correlated with tumor mass in non-small cell lung cancer. J Breath Res. 2014;8:027110. doi: 10.1088/1752-7155/8/2/027110. [DOI] [PubMed] [Google Scholar]
- 11.Dassanayake DL, Muthunayake TM, Senevirathna KH, Siribaddana A. Staging of lung cancer in a tertiary care setting in Sri Lanka, using TNM 7th edition. A comparison against TNM6. BMC Res Notes. 2012;5:143. doi: 10.1186/1756-0500-5-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fumagalli M, Ferrari F, Luisetti M, Stolk J, Hiemstra PS, Capuano D, Viglio S, Fregonese L, Cerveri I, Corana F, et al. Profiling the proteome of exhaled breath condensate in healthy smokers and COPD patients by LC-MS/MS. Int J Mol Sci. 2012;13:13894–13910. doi: 10.3390/ijms131113894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu HC, Lu MC, Lin YC, Wu TC, Hsu JY, Jan MS, Chen CM. Differences in IL-8 in serum and exhaled breath condensate from patients with exacerbated COPD or asthma attacks. J Formos Med Assoc. 2014;113:908–914. doi: 10.1016/j.jfma.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Lee AL, Button BM, Denehy L, Roberts S, Bamford T, Mu FT, Mifsud N, Stirling R, Wilson JW. Exhaled breath condensate pepsin: Potential noninvasive test for gastroesophageal reflux in COPD and bronchiectasis. Respir Care. 2015;60:244–250. doi: 10.4187/respcare.03570. [DOI] [PubMed] [Google Scholar]
- 15.Corhay JL, Moermans C, Henket M, Nguyen Dang D, Duysinx B, Louis R. Increased of exhaled breath condensate neutrophil chemotaxis in acute exacerbation of COPD. Respir Res. 2014;15:115. doi: 10.1186/s12931-014-0115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stefanska J, Sarniak A, Wlodarczyk A, Sokolowska M, Doniec Z, Bialasiewicz P, Nowak D, Pawliczak R. Hydrogen peroxide and nitrite reduction in exhaled breath condensate of COPD patients. Pulm Pharmacol Ther. 2012;25:343–348. doi: 10.1016/j.pupt.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Xiao P, Chen JR, Zhou F, Lu CX, Yang Q, Tao GH, Tao YJ, Chen JL. Methylation of P16 in exhaled breath condensate for diagnosis of non-small cell lung cancer. Lung Cancer. 2014;83:56–60. doi: 10.1016/j.lungcan.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Gessner C, Kuhn H, Toepfer K, Hammerschmidt S, Schauer J, Wirtz H. Detection of p53 gene mutations in exhaled breath condensate of non-small cell lung cancer patients. Lung Cancer. 2004;43:215–222. doi: 10.1016/j.lungcan.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 19.Zou Y, Wang L, Zhao C, Hu Y, Xu S, Ying K, Wang P, Chen X. CEA, SCC and NSE levels in exhaled breath condensate-possible markers for early detection of lung cancer. J Breath Res. 2013;7:047101. doi: 10.1088/1752-7155/7/4/047101. [DOI] [PubMed] [Google Scholar]
- 20.Okamura K, Takayama K, Izumi M, Harada T, Furuyama K, Nakanishi Y. Diagnostic value of CEA and CYFRA 21-1 tumor markers in primary lung cancer. Lung Cancer. 2013;80:45–49. doi: 10.1016/j.lungcan.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Cedrés S, Nuñez I, Longo M, Martinez P, Checa E, Torrejón D, Felip E. Serum tumor markers CEA, CYFRA21-1 and CA-125 are associated with worse prognosis in advanced non-small-cell lung cancer (NSCLC) Clin Lung Cancer. 2011;12:172–179. doi: 10.1016/j.cllc.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012;76:138–143. doi: 10.1016/j.lungcan.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Zhang WM, Zhou J, Ye QJ. Endothelin-1 enhances proliferation of lung cancer cells by increasing intracellular free Ca2+ Life Sci. 2008;82:764–771. doi: 10.1016/j.lfs.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Carpagnano GE, Foschino-Barbaro MP, Resta O, Gramiccioni E, Carpagnano F. Endothelin-1 is increased in the breath condensate of patients with non-small-cell lung cancer. Oncology. 2004;66:180–184. doi: 10.1159/000077992. [DOI] [PubMed] [Google Scholar]
- 25.Boldrini L, Gisfredi S, Ursino S, Lucchi M, Melfi F, Mussi A, Basolo F, Fontanini G. Tumour necrosis factor-alpha: Prognostic role and relationship with interleukin-8 and endothelin-1 in non-small cell lung cancer. Int J Mol Med. 2006;17:887–892. [PubMed] [Google Scholar]


