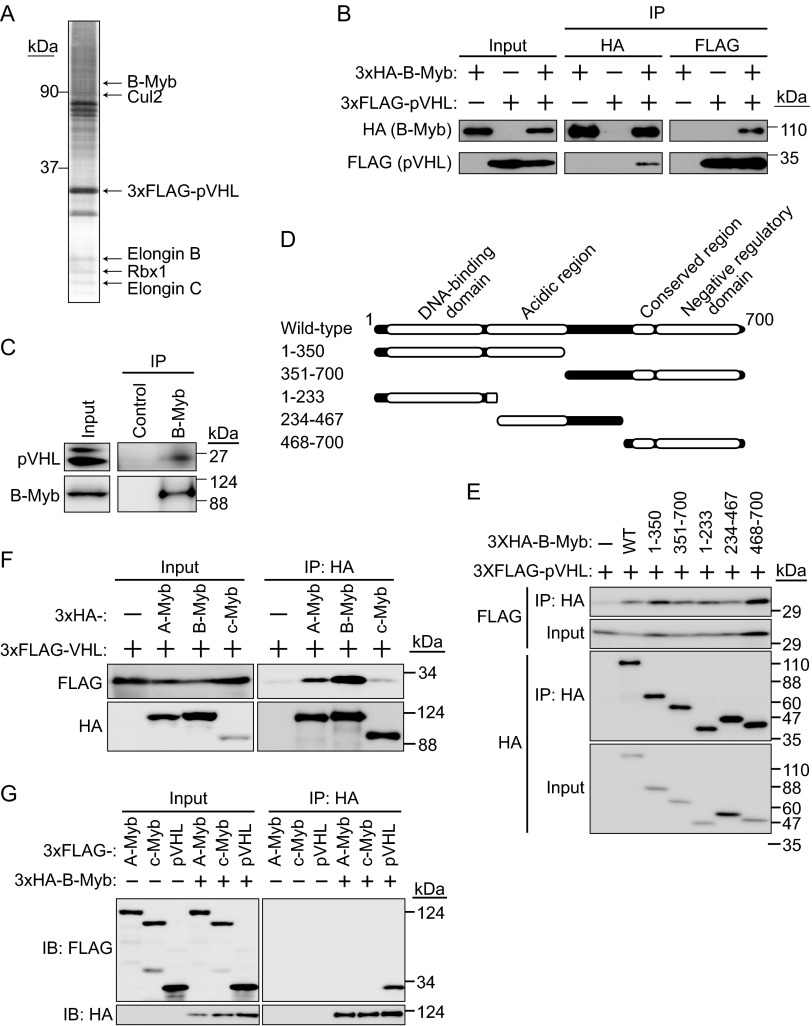
FIG 1.
Interaction between pVHL and B-Myb. (A) Purification of the CRL2pVHL complex. The 3×FLAG-pVHL expressed in 293T cells was purified using anti-FLAG antibody and resolved by SDS-PAGE. In-gel digestion by trypsin was performed for mass spectrometry analysis. A silver-stained SDS-PAGE gel is shown, and the protein bands identified are indicated. B-Myb was identified as a novel protein interacting with pVHL. (B) Interaction between 3×HA–B-Myb and 3×FLAG-pVHL. HEK293T cells expressing 3×HA–B-Myb or 3×FLAG-pVHL (as indicated) were cultured in the presence of the proteasome inhibitor MG132 (10 μM for 6 h), immunoprecipitated (IP) with anti-HA or anti-FLAG antibody, and immunoblotted (IB) with anti-HA or anti-FLAG antibody. (C) Interaction between endogenous pVHL and B-Myb. HEK293T cells were cultured in the presence of MG132 (10 μM for 6 h), immunoprecipitated with anti-B-Myb antibody, and immunoblotted with anti-pVHL or anti-B-Myb antibody. (D) Schematic representation of B-Myb mutants used in this study. (E) Interaction between pVHL and B-Myb via several binding sites. The wild type (WT) or a deletion mutant of 3×HA–B-Myb as indicated was coexpressed with 3×FLAG-pVHL in HEK293T cells. Lysates were immunoprecipitated with anti-HA antibody and immunoblotted with anti-FLAG or anti-HA antibody. (F) Interaction between Myb family proteins and pVHL. HEK293T cells expressing 3×HA–A-Myb, 3×HA–B-Myb, 3×HA–c-Myb, or 3×FLAG-pVHL (as indicated) were cultured in the presence of MG132 (10 μM for 6 h), immunoprecipitated with anti-HA antibody, and immunoblotted with anti-HA or anti-FLAG antibody. (G) Assessment of the interaction between B-Myb and A-Myb or c-Myb. HEK293T cells expressing 3×FLAG–A-Myb, 3×FLAG–c-Myb, 3×FLAG-pVHL, or 3×HA–B-Myb (as indicated) were cultured in the presence of MG132 (10 μM for 6 h), immunoprecipitated with anti-HA antibody, and immunoblotted with anti-HA or anti-FLAG antibody.