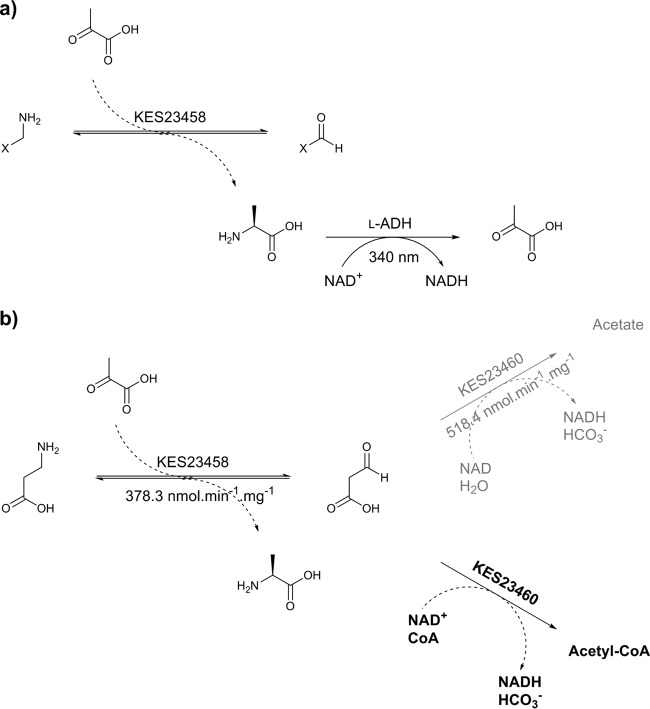
FIG 1.
(a) Alanine dehydrogenase-coupled UV assay for transaminase activity. The transaminase catalyzes the amine transfer from the substrate to pyruvate (cosubstrate), producing alanine as a coproduct. The dehydrogenase then catalyzes the oxidative deamination of alanine utilizing a cofactor, NAD+ (NAD), which is concurrently reduced to NADH. Formation of NADH can be detected by UV photospectrometry as a hyperchromic shift at 340 nm. (b) Analogous assays performed with KES23460. In this case the transaminase reaction shown is the β-alanine/pyruvate transamination reaction. If CoA is present, malonate semialdehyde produced by transamination is then converted by KES23460 to acetyl-CoA in an NAD+ dependent reaction (shown in bold). Without CoA present malonate semialdehyde is converted to acetate and bicarbonate (shown in gray). Both of these reactions require the conversion of NAD+ to NADH, which can be followed by UV photospectrometry at 340 nm. Based on spectrophotometric data, and the value of acetyl-CoA as a metabolic intermediate, the CoA-dependent pathway is likely to be the physiologically relevant reaction for these genes.