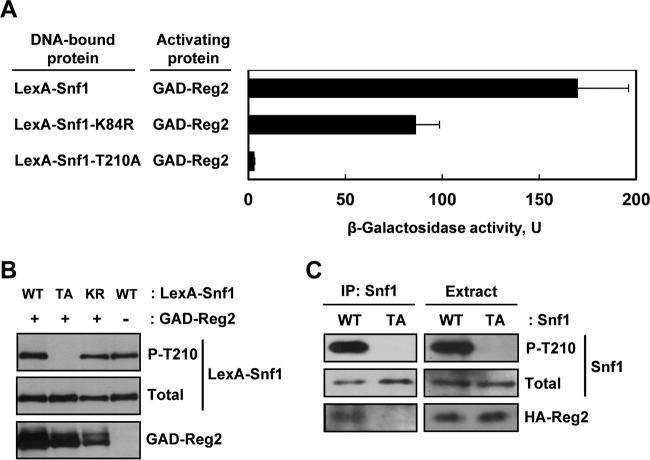
FIG 2.
Mutation of the Snf1 activation loop threonine abolishes interaction with Reg2. (A) Wild-type strain MKY343 carrying a lexAop-lacZ reporter (plasmid pSH18-18) and expressing the indicated protein pairs was grown to mid-log phase in selective SC medium lacking uracil, histidine, and leucine and containing abundant (2%) glucose and then shifted for 3 h to an otherwise identical medium containing limiting (0.05%) glucose. β-Galactosidase activity was assayed in permeabilized cells and expressed in Miller units. Shown are the results for limiting glucose; the values are averages for five transformants, and the error bars indicate standard errors. Under glucose-rich conditions, the values were 2.5 U or lower. (B) Representative transformants were cultured under glucose-limiting conditions as for panel A, and the levels of LexA-Snf1 phosphorylated at Thr210 (P-T210), total LexA-Snf1 (Total), and GAD-Reg2 were confirmed by immunoblotting as described in Materials and Methods. WT, LexA-Snf1; TA, LexA-Snf1-T210A; KR, LexA-Snf1-K84R. The cells also expressed GAD-Reg2 (GAD-Reg2, +) or carried the corresponding vector pACTII (GAD-Reg2, −). (C) Coimmunoprecipitation assays. Cells of strain MKY362 (snf1Δ) transformed with combinations of plasmids expressing HA-Reg2 and wild-type Snf1 (WT) or nonphosphorylatable Snf1-T210A (TA) were grown to mid-log phase in selective SC medium lacking leucine and uracil and containing abundant (2%) glucose and then shifted to an otherwise identical medium containing limiting (0.05%) glucose for 15 min. Following the shift to limiting glucose, protein extracts were prepared, and Snf1 was immunoprecipitated with anti-polyhistidine antibody. The immunoprecipitates (IP: Snf1) and extracts (Extract) were examined by immunoblotting for the presence of phospho-Thr210-Snf1 (P-T210), total Snf1 protein (Total), and HA-Reg2.