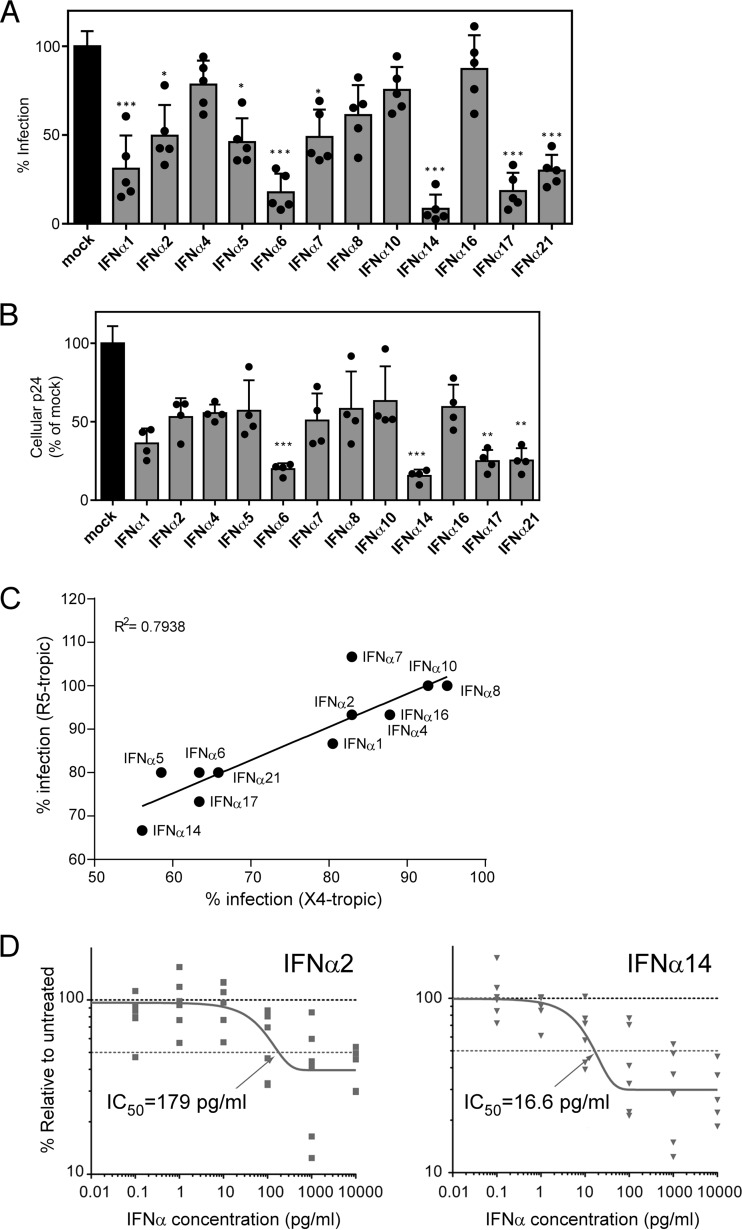
FIG 1.
Inhibition of HIV-1 replication by IFN-α subtypes in vitro. (A and B) The infectivity of supernatants harvested from PBMCs exposed to X4-tropic HIV-1NL4-3_IRES_eGFP in the presence of different human IFN-α subtypes for 2 days was determined. (A) The supernatants were incubated with TZM-bl reporter cells, and β-galactosidase activity was compared to that of untreated supernatants after 3 days of incubation. (B) Additionally, the cells were lysed, and the cellular p24 protein content was analyzed by ELISA. Five healthy donors were used for the IFN-α inhibition assay, and the TZM-bl assay was measured in triplicate. Mean values plus standard errors of the mean (SEM) are shown, and individual donors are represented by dots. ***, P < 0.001; **, P < 0.01; *, P < 0.05; determined by one-way analysis of variance (ANOVA) with Dunnett's posttest. (C) The infectivities of IFN-α-treated X4- and R5-tropic viruses relative to untreated PBMCs were determined and compared using a Pearson correlation test. (D) Dose-response inhibition in mucosal immune cells. LPMCs (n = 6 donors) were infected with HIV-1BaL, resuspended with various doses of IFN-α2 and IFN-α14 (or mock infected), and evaluated for intracellular Gag p24+ expression in CD3+ CD8− cells at 4 dpi by flow cytometry. The data were normalized against mock infection for each donor. The dose-response curves were generated using a one-phase decay equation that was also used to evaluate the IC50.