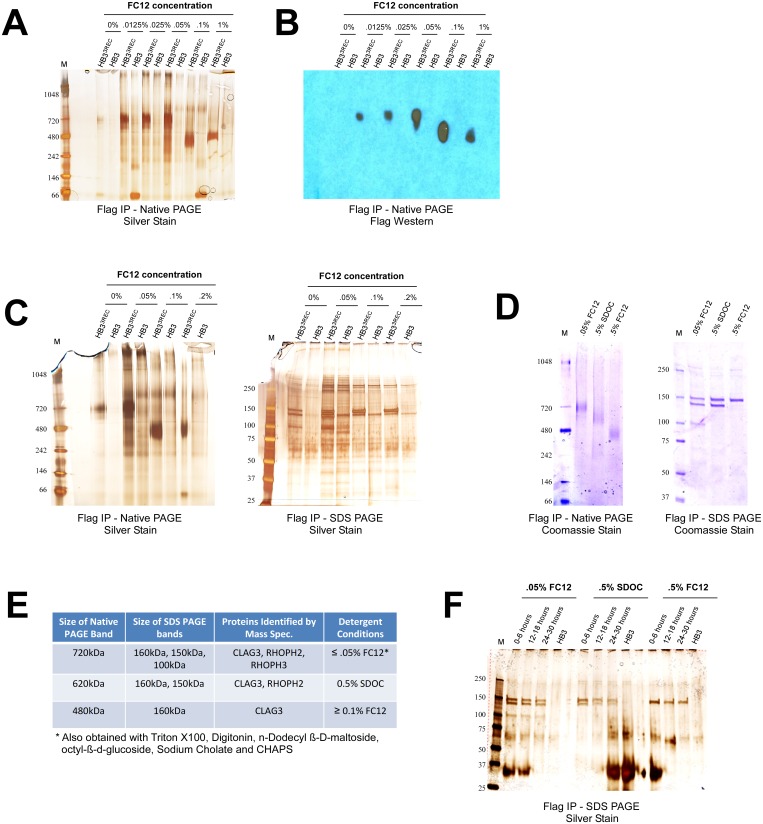
Fig 2. CLAG3 forms high molecular weight membrane-associated complexes.
(A) Blue Native PAGE analysis of FLAG IP eluates obtained from a FLAG-tagged CLAG3 parasite (HB33REC) and an isogenic untagged HB3 negative control [8]. Two distinct bands can be seen in HB33REC that are absent in HB3: a ~720kDa band for concentrations of FC12 ≤ 0.05% and a ~480kDa band for concentrations of FC12 ≥ 0.1%. (B) FLAG Western blot confirms that the 720kDa and 480kDa bands contain CLAG3. (C) SDS PAGE analysis shows that upon addition of SDS and 2-mercaptoethanol the 720kDa complex dissociates to three proteins (CLAG3, RHOPH2, and RHOPH3 as determined by mass spectrometry), while the 480kDa complex dissociates to only one protein (CLAG3). (D) Sodium deoxycholate (SDOC) at 0.5% (w/v) yields an intermediate ~620kDa Native PAGE band that contains CLAG3 and RHOPH2, but not RHOPH3 (as determined by SDS PAGE and mass spectrometry). (E) A summary of the mass spectrometry results for the indicated Native and SDS PAGE bands. (F) IP experiments on tightly synchronized parasites show that CLAG3, RHOPH2 and RHOPH3 appear to associate faithfully in early (0–6 hours post-invasion), mid-stage (12–16 hours post-invasion), or late rings/early trophozoites (24–30 hours post-invasion). The prominent 30kDa band corresponds to light chain IgG.