Abstract
Background and Objective
Atherosclerosis is both a chronic inflammatory disease and a lipid metabolism disorder. C/EBPβ is well documented for its role in the development of hematopoietic cells and integration of lipid metabolism. However, C/EBPβ's role in atherosclerotic progression has not been examined. We assessed the impact of hematopoietic CEBPβ deletion in ApoE−/− mice on hyperlipidemia, inflammatory responses and lesion formation in the aorta.
Methods and Results
ApoE−/− mice were reconstituted with bone marrow cells derived from either WT or C/EBPβ−/− mice and placed on low fat or high fat/high cholesterol diet for 11 weeks. Hematopoietic C/EBPβ deletion in ApoE−/− mice reduced blood and hepatic lipids and gene expression of hepatic stearoyl CoA desaturase 1 and fatty acid synthase while expression of ATP binding cassette transporter G1, cholesterol 7-alpha-hydroxylase, and liver X receptor alpha genes were significantly increased. ApoE−/− mice reconstituted with C/EBPβ−/− bone marrow cells also significantly reduced blood cytokine levels and reduced lesion area in aortic sinuses compared with ApoE−/− mice reconstituted with WT bone marrow cells. Silencing of C/EBPβ in RAW264.7 macrophage cells prevented oxLDL-mediated foam cell formation and inflammatory cytokine secretion in conditioned medium.
Conclusion
C/EBPβ in hematopoietic cells is crucial to regulate diet-induced inflammation, hyperlipidemia and atherosclerosis development.
Keywords: Atherosclerosis, Bile acid, Cytokine, Hematopoietic stem cell, Inflammation, Macrophage foam cells, Cholesterol efflux
1. Introduction
Macrophages play a central role in atherogenesis through the accumulation of cholesterol and the production of inflammatory mediators and cytokines. A critical step in the development of atherosclerosis is the accumulation of cholesterol-laden macrophages, which in the arterial wall is the characteristic of the early atherosclerotic lesion [1,2]. Cellular cholesterol content in macrophages is determined by uptake (mediated by scavenger receptors) and efflux of cholesterol (mediated by cholesterol acceptors) [3], an imbalance of which results in the formation of foam cells, which in turn promote lipid deposition and lesion growth.
The modulation of macrophage accumulation and function represents an appealing therapeutic target for the treatment and prevention of inflammation in a variety of disease pathways. Inflammatory pathways in macrophages are under stringent control by a variety of transcription factors and coregulatory molecules. Among these are NF-κB, AP1, the PPAR family, liver X receptor alpha (LXRα/Nr1h3), and their associated coactivators and corepressors [4]. However, the signaling pathways that regulate inflammation and trigger foam cell formation and cytokine production in atherosclerosis remain incompletely understood.
C/EBPβ is a member of the CCAAT/enhancer binding protein family of basic leucine zipper transcription factors with classical functions in transcriptional and translational regulation of lipid metabolism in the liver and adipose tissue [5,6]. In addition, C/EBPβ has been described as a macrophage lineage determination factor, also referred to as a pioneer factor or master regulator because it represents a placeholder that can be replaced by other transcription factors at later stages during development [7]. Beyond its role in early myeloid and adipose cell development [8–10], we demonstrated that adenovirus delivery of C/EBPβ to the liver of WT mice re-capitulated many of the nonalcoholic steatohepatitis-like phenotypes including hepatic inflammation, endoplasmic reticulum stress, and lipid accumulation [6]. Conversely, we showed that C/EBPβ deletion in Leprdb/db mice reduced adiposity, hepatic steatosis, and diabetes [5]. More recently, we showed that hematopoietic deletion of C/EBPβ reduced obesity-linked inflammation and insulin resistance in WT mice [11], suggesting that C/EBPβ deletion may be important in controlling innate immunity.
Because C/EBPβ is known to play an important role in inflammation [9], we hypothesized that hematopoietic deletion of C/EBPβ in hyperlipidemic ApoE−/− mice [12] would reduce inflammation and hyperlipidemia, and thereby interrupt processes important for development of atherosclerosis. Surprisingly, our data demonstrate that C/EBPβ deletion in hematopoietic cells had pleiotropic effects on lipids and the evolution of atherosclerosis in ApoE−/− mice including reduced total and LDL cholesterol, along with modulation of genes implicated in triglyceride and cholesterol metabolism. Furthermore, adipose tissue inflammation, circulatory cytokine levels, and atherosclerotic lesion formation in the aortic sinuses of chimeric ApoE−/− mice were suppressed. We documented that shRNA-mediated deletion of C/EBPβ in RAW264.7 macrophages attenuated oxidized LDL (oxLDL)-mediated foam cell formation and secretion of inflammatory cytokines in the medium. Altogether, these findings indicate that C/EBPβ in hematopoietic-derived cells regulates cholesterol balance in the macrophage and liver and therefore is a crucial transcriptional regulator of diet-induced inflammation, hyperlipidemia, and atherosclerosis development.
2. Materials and methods
2.1. Animals & bone marrow transplants
All animal care and procedures were conducted according to the policies on animal welfare of the National Institute of Health and were approved by the Institutional Animal Care and Use Committee of the University of Colorado Denver and Texas Tech University. Every effort was made to minimize the animal stress and suffering.
Six-to eight-week old B6.SJL (CD45.1; #002104; Jackson Laboratory, Bar Harbor, ME) ApoE−/− female recipient mice were subjected to a total of 1000 rad (2 × 500 rad 3 h apart) of whole body irradiation to eliminate endogenous bone marrow stem cells. Bone marrow was harvested from donor mice (8–10 week old) by flushing the femur of WT C57BL/6 (CD45.2) and C/EBPβ−/− (CD45.2) with Hank's buffer, as described previously [11]. Recipient mice were then anesthetized with isoflurane and injected with 2.5 × 106 cells into the retro-orbital sinus cavity. After a 4 week recovery, mice were placed on either low fat (LF; 10 kcal% fat, D12102; Research Diets, New Brunswick, NJ) or high fat/high cholesterol diet (HF/HC; 40 kcal% fat, 1.25% cholesterol, 0% cholic acid; D12108C; Research Diets) for 11 weeks (n = 6). Food intake and body weight were measured weekly. To test for engraftment of donor bone marrow, at 10 weeks post-irradiation mice were anesthetized with isoflurane and blood was collected via retro-orbital sinus and analyzed by flow cytometry for CD45.1 or CD45.2 positive cells as described previously [11]. ApoE−/− mice with engraftment of >85% donor cells were continued on the experimental diets and used for final analyses. At the end of the 11 week diet treatment, tissues were collected in mice anesthetized with isoflurane after a 6-h fast. Tissues were immediately placed in tubes with RNAlater (Qiagen, Valencia, CA) or snap-frozen in liquid nitrogen. Blood samples were collected after a 6-h daytime fast from the vena cava.
2.2. Measurement of serum metabolites
For glucose tolerance tests (GTT), ApoE−/− mice transplanted with WT and C/EBPβ−/− bone marrow cells and fed LF and HF/HC diets were fasted for 4 h and injected intraperitoneally with glucose (2 mg/g body weight), as described previously [11,13]. Blood samples were collected from the tail vein at 0, 30, 60, 90 or 120 min and glucose was measured using a glucometer. Area under the curve (AUC) of glucose was calculated as described previously [14]. To circumvent influences of glucose injection on hepatic gene expression, animals were sacrificed one week after the last GTT, i.e. after 11 weeks on the experimental diets. Serum insulin and adiponectin levels were measured by ELISA kits from ALPCO (Windham, NH). Serum cytokines (IL-1β, TNFα, MCP1, and IL-6) was measured by multiplex assay (Bio-Rad, Hercules, CA). Serum total cholesterol, triglycerides, HDL cholesterol, and non-HDL cholesterol were analyzed enzymatically using a Beckman Coulter AU automated chemistry analyzer (Beckman Coulter, Brea, CA).
2.3. Analysis of liver lipids
Lipids were extracted from about 50 mg of frozen liver tissue using a Folch method [15]. Half of the lipid extract was saponified and total cholesterol was extracted as previously described [16]. Cholesterol and stigmasterol (internal standard) were measured using an HPLC system (Waters Corp., Milford, MA) equipped with a C18 reverse-phase column (150 mm × 4.6 mm, 5 µm size). The mobile phase was methanol at 1 mL/min. Detection was monitored at 210 nm by a Waters 2489 UV–visible detector. The tissue pellet was digested by 1 N NaOH and total protein was measured using a BCA kit. Total cholesterol was expressed as mg of cholesterol per mg of protein.
2.4. Evaluation of lesion size
Atherosclerotic lesions at the aortic sinuses were analyzed as described previously after 11 weeks of diet treatment [17]. The upper portion of the heart (aortic sinuses) was obtained, embedded in OCT compound, and stored at −80 °C. Ten µm sections were analyzed for a distance of 800 µm. Sections were stained with Oil Red O. The lipid-staining areas were determined in a blinded fashion by light microscopy. The mean value of lesion area of aortic wall per section was then calculated.
2.5. Quantitative real-time PCR
Total RNA isolation, reverse transcription, and quantitative real-time PCR (qPCR) were performed as described previously [11]. For primers used in qPCR, see Table S1.
2.6. Culture and treatment of RAW264.7 macrophage cells
RAW264.7 macrophage cells were obtained from ATCC (Manassas, VA) and cultured in modified DMEM (4.5 g glucose/L) as described before [11]. The macrophage cells (70–80% confluent) were infected with control (50 pfu/cell) and C/EBPβ-shRNA (50 pfu/cell) for 24 h followed by treatment with nLDL and oxLDL (20 µg/mL; Biomedical Technologies, Inc., Stoughton, MA) for an additional 24 h. Conditioned medium was collected for protein array. Cells were fixed and stained with Oil Red O to detect lipid accumulation. In some experiments, cells were harvested for RNA isolation and Western blot.
2.7. Protein array and immunoblot analysis
Comprehensive analysis of cytokine levels in the conditioned media from RAW264.7 macrophage cell culture was performed using commercially available Mouse Inflammation Antibody Array 1.1 (RayBiotech, Inc., Norcross, GA) according to manufacturer's protocol. Cytosolic and nuclear extracts were prepared from RAW264.7 macrophage cells and subjected to Western blot analysis for P-JNK and C/EBPβ, respectively, as previously described [11]. Primary antibodies used in this study were C/EBPβ and actin (Santa Cruz Biotechnology, Santa Cruz, CA) and P-JNK (Cell Signaling Technology, Danvers, MA).
2.8. Statistical analysis
Statistical comparisons between groups were made either using Student's t-test, or two-way ANOVA, followed by Tukey's post hoc test. GTT values were analyzed using two-way repeated measures ANOVA. All values are reported as mean ± standard error. Differences were considered to be statistically significant at P values 0.05 or less.
3. Results
3.1. Hematopoietic deletion of C/EBPβ reduced pro-inflammatory cytokines in serum and adipose tissue of ApoE−/− mice
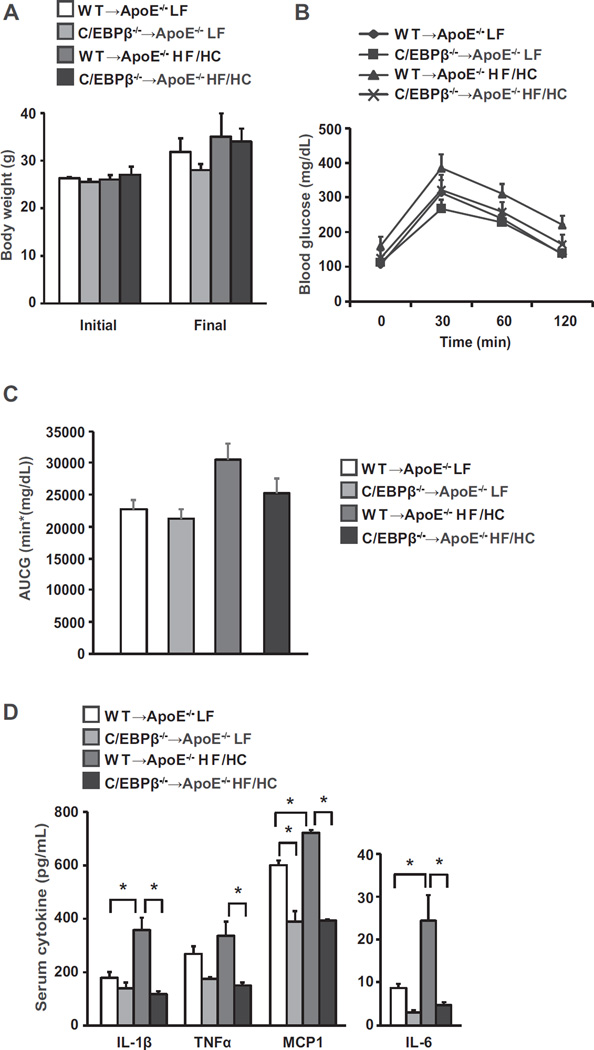
C/EBPβ plays an important role in the generation and activation of resident macrophages [10]. Although C/EBPβ is induced by inflammatory stimuli and affects cytokine production in several macrophage types [18,19], its role in atherosclerosis is unknown. We therefore evaluated the impact of hematopoietic C/EBPβ deletion from bone marrow on mechanisms that provoke atherosclerosis in ApoE−/− mice. ApoE−/− mice transplanted with WT or C/EBPβ−/− bone marrow cells were placed on high fat/high cholesterol diet (HF/HC) or low fat diet (LF) for 11 weeks. Mice on HF/HC diets gained more weight than animals on LF diet (Fig. 1A) but the difference was not statistically significant. Final body weights were similar between WT → ApoE−/− HF/HC and C/EBPβ−/− → ApoE−/− HF/HC mice (Fig. 1A). We also assessed glucose tolerance in the bone marrow transplanted mice. Animals on the HF/HC diet had significantly higher GTT values at baseline than those on a LF diet (P = 0.023; Fig. 1B). Area under the curve (AUC) analyses of the GTT curves for the four groups showed differences for LF versus HF/HC diets when using uncorrected AUCs (P = 0.006) but not when correcting for the baseline (P = 0.57, Fig. 1C). WT → ApoE−/− versus C/EBPβ−/− → ApoE−/− was not significantly different in any of the aforementioned analyses (P > 0.07 in all cases). There was no evidence in support of an ApoE by-diet interaction in the AUC analyses. Fasting plasma insulin level was similar between the HF/HC groups (data not shown).
Fig. 1.
ApoE−/− mice transplanted with WT or C/EBPβ−/− bone marrow cells were fed either a LF or high fat/high cholesterol (HF/HC) diet for 11 weeks as described in Materials and Methods (n = 4–5 per group). Initial and final body weight (A) and GTT results (B). C: AUC of glucose. GTT values were analyzed by two-way repeated measures ANOVA. Data are presented as mean ± SEM. D: Inflammatory cytokines in serum. Data are presented as mean ± SEM. *P < 0.05 by two-way ANOVA followed by Tukey's post hoc test.
Elevated levels of plasma inflammatory cytokines, and chemokines, have been implicated in the initiation and progression of cardiovascular disease [20,21]. As expected, HF/HC diet significantly increased IL-1β, MCP1, and IL-6 cytokine levels in WT → ApoE−/− mice compared with WT → ApoE−/− LF mice (Fig. 1D). By contrast, hematopoietic deletion of C/EBPβ−/− produced significant reductions (45–81%) in IL-1β, TNFα, MCP1, and IL-6 cytokine levels in C/EBPβ−/− → ApoE−/− HF/HC mice compared with WT → ApoE−/− HF/HC mice (Fig. 1D).
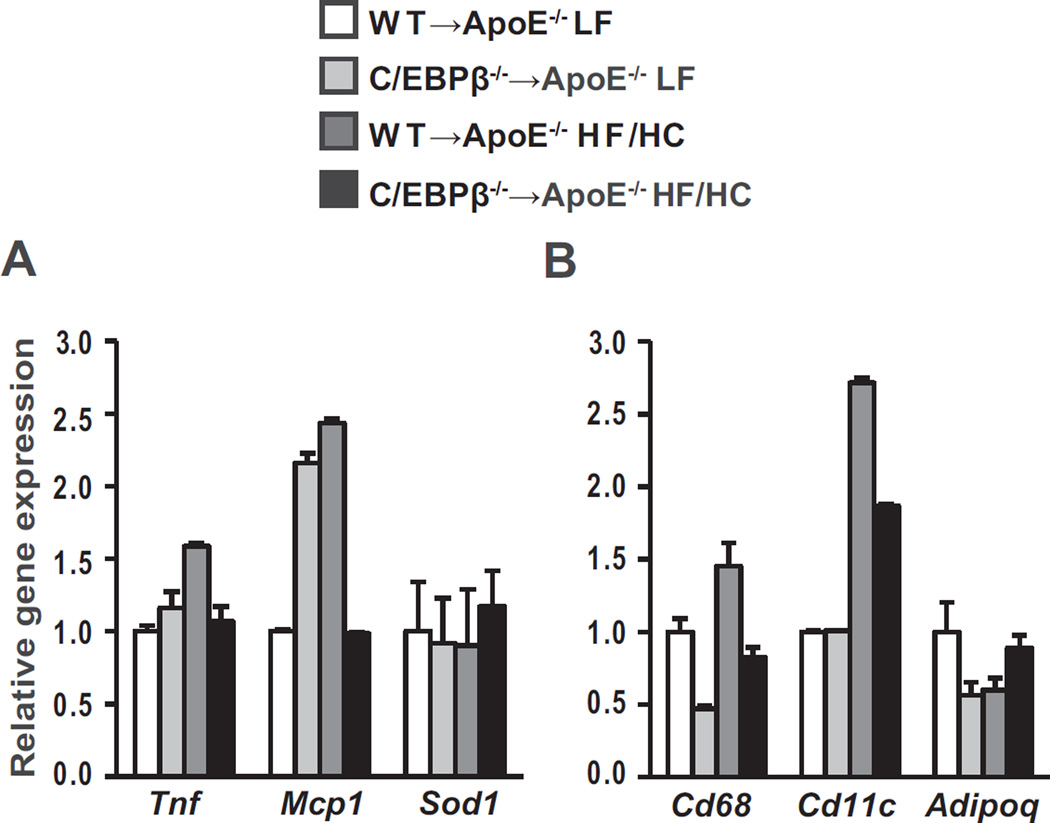
The systemic inflammation that is associated with atherosclerosis is partly derived from adipose tissue [22,23]. In our previous study we found that hematopoietic C/EBPβ deletion in wildtype mice prevented high fat diet-mediated induction of adipose tissue inflammation [11]. To investigate whether adipose tissue was the source of reduced circulating cytokines, we determined the effect of hematopoietic C/EBPβ deletion on adipose tissue inflammation in C/EBPβ−/− → ApoE−/− mice under LF and HF/HC fed conditions. HF/HC diet resulted in a non-significant increase in Tnf and Mcp1 gene expression in WT → ApoE−/− mice compared with LF diet-fed mice (Fig. 2A). Interestingly, despite similar weight gain on the HF/HC diet, we found reductions in Tnf and Mcp1 gene expression in adipose tissue of C/EBPβ−/− → ApoE−/− HF/HC mice compared with WT → ApoE−/− HF/HC mice although the differences were not significant (Fig. 2A). The increase in adipose tissue inflammation is associated with recruitment of macrophages in adipose tissue and may further increase the magnitude of inflammation [24,25]. Consistent with reduced expression of inflammatory genes in adipose tissues of C/EBPβ−/− → ApoE−/− mice, macrophage marker genes Cd68 and Cd11c showed non-significant reductions in the WT → ApoE−/− compared with C/EBPβ−/− → ApoE−/− mice on HF/HC diet (Fig. 2B).
Fig. 2.
Gene expression analyzed by qPCR in epididymal adipose tissue from ApoE−/− mice reconstituted with C/EBPβ−/− or WT bone marrow cells (A-B; n = 4 per group). Data are presented as mean ± SEM and analyzed by two-way ANOVA followed by Tukey's post hoc test.
3.2. A more anti-atherogenic lipoprotein profile in ApoE−/− mice transplanted with C/EBPβ−/− bone marrow cells
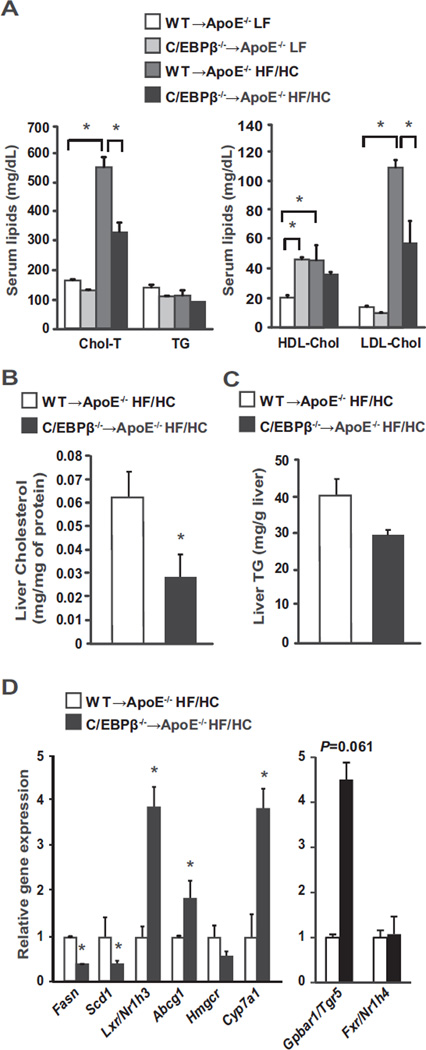
An increase in serum and tissue lipids is considered a critical factor in the development of atherosclerosis [26,27]. We assessed the effects of reconstitution with bone marrow lacking C/EBPβ on serum lipid levels in ApoE−/− mice. On LF diet, total cholesterol levels were similar in ApoE−/− mice transplanted with C/EBPβ−/− bone marrow cells (Fig. 3A). However, HF/HC diet significantly increased serum cholesterol (P < 0.0001), HDL cholesterol (P = 0.033) and LDL cholesterol (P < 0.0001) in WT → ApoE−/− mice (Fig. 3A). By contrast, hematopoietic deletion of C/EBPβ in ApoE−/− mice blunted the HF/HC diet-induced increase in serum total and LDL cholesterol (P < 0.0001, P = 0.0002, respectively; Fig. 3A). In the liver of ApoE−/− mice with C/EBPβ deletion in hematopoietic cells, total cholesterol levels were reduced 60% (Fig. 3B) while liver triglyceride levels appeared to be lower in C/EBPβ−/− → ApoE−/− HF/HC mice compared with WT → ApoE−/− HF/HC mice but was not statistically significant (Fig. 3C). Overall, these data showed that C/EBPβ−/− → ApoE−/− HF/HC mice attenuated serum and liver lipid profiles compared with WT → ApoE−/− HF/HC mice.
Fig. 3.
Lipoprotein profiles and gene expression in liver from ApoE−/− mice transplanted with WT or C/EBPβ−/− bone marrow cells (n = 4–5 per group). A: Serum lipid analysis for total cholesterol (Chol-T), triglyceride (TG), HDL cholesterol (HDL-Chol) and LDL cholesterol (LDL-Chol). Data are presented as mean ± SEM. *P < 0.05 by two-way ANOVA followed by Tukey's post hoc test. Cholesterol (B) and triglyceride (TG; C) levels in liver. *P < 0.05 by Student's t tests. D: Gene analyses in liver tissue. B–D: Data are presented as mean ± SEM. *P < 0.05 versus WT → ApoE−/− HF/HC group as tested by Student's t-test.
3.3. Hematopoietic C/EBPβ deletion in ApoE−/− mice reduced Fasn and Scd1 gene expression but increased Abcg1, Lxr/Nr1h3, and Gpbar1/Tgr5 gene expression in liver
Atherosclerosis is characterized by a dysfunction of hepatic lipid metabolism [28,29]. In order to investigate the possible mechanisms regarding the observed differences in serum and liver lipid levels, we quantified mRNA expression levels of key liver enzymes in fatty acid and triglyceride synthesis and cholesterol efflux. Expression of fatty acid synthase (Fasn) and stearoyl-CoA desaturase (Scd1) were reduced (40–50%), while expression of Lxr/Nr1h3, a gene that increases cholesterol metabolism, was significantly increased fourfold in liver along with its target gene ATP-binding cassette transporter G1 (Abcg1) in C/EBPβ−/− → ApoE−/− HF/HC mice compared with WT → ApoE−/− HF/HC mice (Fig. 3D). HMG-CoA reductase (Hmgcr), a gene that controls cholesterol synthesis, was slightly lower in C/EBPβ−/− → ApoE−/− HF/HC mice (P = 0.14), and cholesterol 7 alpha-hydroxylase (Cyp7a1), which enhances cholesterol to bile acid conversion and plays a crucial role in regulation of serum cholesterol levels, was induced fourfold compared with WT → ApoE−/− HF/HC mice (Fig. 3D). We also analyzed the expression of bile acid sensors farnesoid X receptor (Fxr/Nr1h4) and G protein-coupled bile acid receptor 1 (Gpbar1/Tgr5) genes, both of which are activated by bile acids and play key roles in hepatic lipid homeostasis and inflammation [30,31]. Fxr/Nr1h4 gene expression was similar between groups but interestingly, the expression of Gpbar1/Tgr5 was over fourfold higher (P = 0.061) in C/EBPβ−/− → ApoE−/− HF/HC mice (Fig. 3D). Overall, these results indicate that C/EBPβ deletion in bone marrow reduced cholesterol levels in liver and serum, and suggests that despite a substantial increase in Lxr/Nr1h3 activation, C/EBPβ in hematopoietic-derived cells is required to induce liver and serum cholesterol levels in ApoE−/− mice.
3.4. Atherosclerosis is dramatically decreased in ApoE−/− mice transplanted with C/EBPβ−/− bone marrow cells
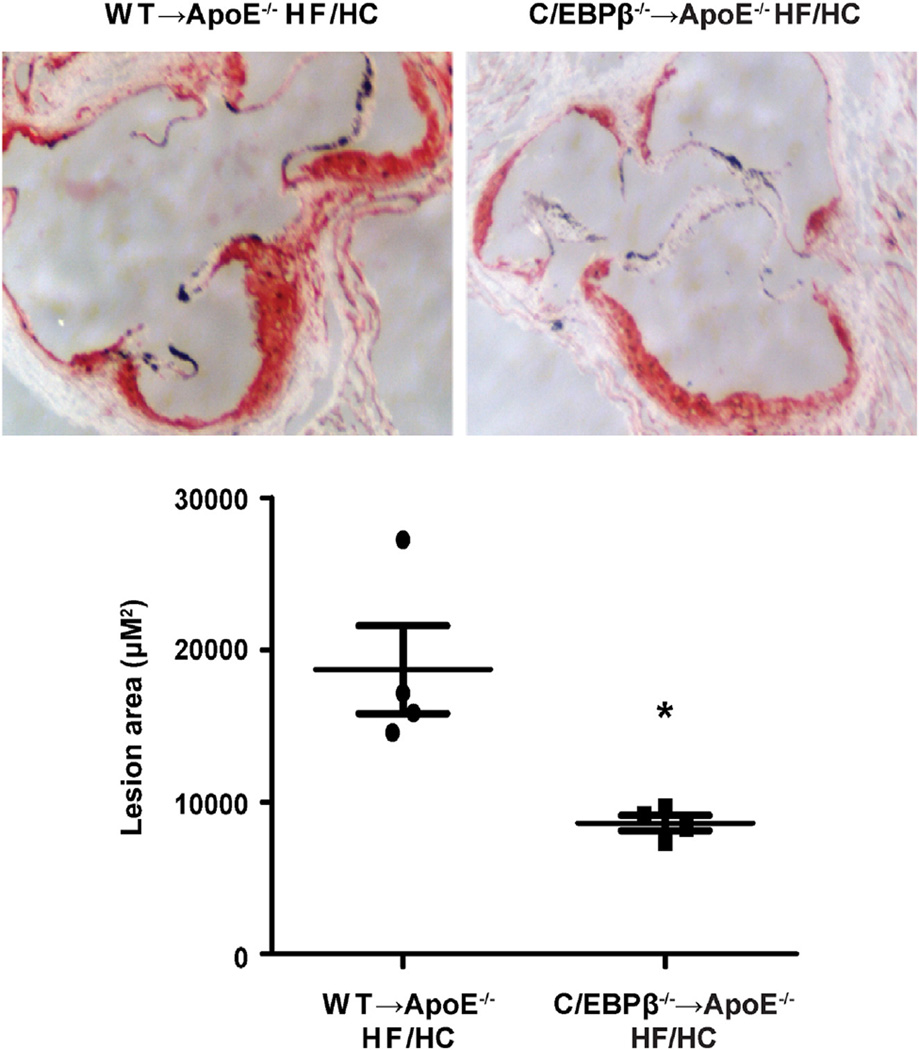
Given the reduction in serum cytokines and lipids, we investigated the impact of C/EBPβ deletion in hematopoietic cells on atherosclerotic lesion development and lesion sizes in the aortic sinuses of ApoE−/− mice. Lesion formation was evaluated by neutral lipid stain Oil Red O and sections of Oil Red O-stained aortic sinuses were quantified after 11 weeks of HF/HC feeding. Quantification of the lesion sizes in Oil Red O-stained sections of the aortic sinuses revealed that deletion of C/EBPβ in macrophages led to a 50% decrease in the mean atherosclerotic lesion size in C/EBPβ−/− → ApoE−/− HF/HC mice compared with lesions in WT → ApoE−/− HF/HC mice (Fig. 4).
Fig. 4.
Oil Red O staining of aortic sinuses and quantitation of lesion area (n = 4 per group). *P < 0.05 versus WT → ApoE−/− HF/HC group as tested by Student's t-test. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.5. C/EBPβ regulates oxLDL-mediated macrophage foam cell formation
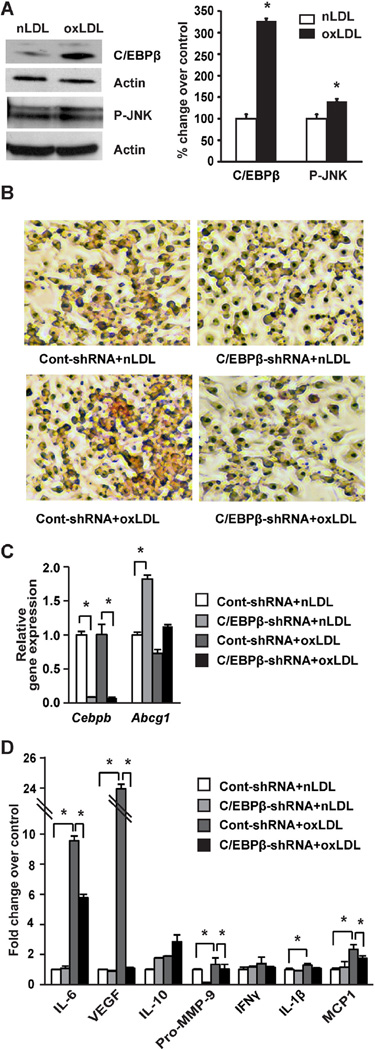
Macrophages play a critical role in the development of atherosclerosis [32]. Accumulation of macrophage foam cells which results from impaired cholesterol metabolism is a prominent early event in the development of atherosclerosis [33,34]. Given the reduction in lipids, inflammation, and aortic lesions in ApoE−/− mice lacking C/EBPβ in bone marrow, we hypothesized that C/EBPβ could have direct effects on macrophage cholesterol balance. Using RAW264.7 cells, we found that macrophage cells treated with oxLDL significantly increased the expression of C/EBPβ protein (Fig. 5A), along with the cell stress marker phosphorylated JNK (P-JNK). To further investigate whether C/EBPβ controls cholesterol balance in macrophages, we examined the effects of oxLDL on cholesterol balance in RAW264.7 cells using C/EBPβ-shRNA knockdown. We found using shRNA-mediated knockdown of C/EBPβ that oxLDL-mediated induction of lipid accumulation was dramatically decreased as evidenced by Oil Red O staining (Fig. 5B). Consistent with this reduction of lipid accumulation, silencing of C/EBPβ moderately increased the expression of Abcg1 gene (Fig. 5C), which is implicated in cholesterol efflux [35,36] but this change was not statistically significant (P = 0.11).
Fig. 5.
RAW264.7 macrophage cell treatment with oxLDL and effects of shRNA knockdown of C/EBPβ. A: RAW264.7 cells were treated with nLDL or oxLDL (20 µg/mL) for 24 h (n = 3 per experiment). Immunoblots and densitometric values for C/EBPβ (in nuclear fraction) and P-JNK (in cytosolic extract), with representative blots shown. Data represent the mean ± SEM, expressed as percent change over control after normalizing to actin. *P < 0.05 versus nLDL group as tested by Student t-test. B–D: RAW macrophage cells were transduced with control-shRNA (50 pfu/cell) or C/EBPβ shRNA (50 pfu/cell) for 24 h followed by treatment with nLDL or oxLDL (20 µg/mL) for an additional 24 h (n = 3 per experiment). B: Cells were fixed and stained with Oil Red O to detect lipid accumulation. C: Gene expression analysis by qPCR in RAW264.7 macrophage cells. Data are presented as mean ± SEM. *P < 0.05 as tested by two-way ANOVA followed by Tukey's post hoc test. D: Protein array data using conditioned medium collected from RAW cell experiments. Data are presented as mean ± SEM. *P < 0.05 as tested by two-way ANOVA followed by Tukey's post hoc test. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.6. Attenuated expression of pro-inflammatory proteins in conditioned medium of C/EBPβ-depleted RAW264.7 macrophages
During atherogenesis, macrophages take up lipid moieties to become foam cells that secrete pro-inflammatory cytokines, which, in turn, propagate lesion formation and further perpetuate vessel wall inflammation [2,37,38]. To gain further insight into C/EBPβ's role in cytokine production in macrophages, we measured relative levels of key inflammatory cytokines in conditioned medium from RAW264.7 macrophages cultured in the absence or presence of C/EBPβ-shRNA and oxLDL. We used a protein array method that quantifies the relative levels of cytokines by immunoblot. oxLDL treatment significantly increased IL-6, VEGF, Pro-MMP-9, IL-1β, and MCP1 protein expression in Cont-shRNA cells compared to nLDL treatment (P < 0.0001, P < 0.0001, P = 0.002, P = 0.021, P < 0.0001, respectively; Fig. 5D). Silencing C/EBPβ in cells treated with oxLDL significantly reduced the expression of IL-6 and VEGF compared with the Cont-shRNA + oxLDL (P = 0.0115 and P < 0.0001, respectively; Fig. 5D). Expression of MCP1 was significantly decreased (P = 0.0008) while IL-1β was slightly decreased (P = 0.091) in C/EBPβ-shRNA + oxLDL compared with the ContshRNA + oxLDL group (Fig. 5D). By contrast, the level of IL-10, an anti-inflammatory cytokine, was moderately higher (P = 0.18) in C/EBPβ-shRNA macrophage cells compared with cells treated with control shRNA and oxLDL (Fig. 5D).
4. Discussion
Given the pro-inflammatory role of C/EBPβ in tissue macrophages, along with the profound reduction in obesity in global C/EBPβ−/− mice [11], we aimed to investigate: 1) if bone marrow deficiency of C/EBPβ can attenuate the local and systemic inflammation pattern seen in the global ApoE−/− mice, and 2) whether the reduction in inflammation resulting from hematopoietic C/EBPβ deletion could attenuate the development of atherosclerosis, a chronic inflammatory disease associated with hyperlipidemia. The present study demonstrated for the first time that C/EBPβ deletion in hematopoietic cells significantly reduced atherosclerotic lesion formation in aortic sinuses of ApoE−/− mice on a high fat/high cholesterol diet. This reduction of lesion formation in ApoE−/− mice was associated with marked reduction in circulating cytokine levels while expression of pro-inflammatory and macrophage marker genes in visceral adipose tissue were moderately decreased. In addition, C/EBPβ deletion in hematopoietic cells also reduced serum total and LDL cholesterol level without affecting the HDL cholesterol levels. These findings suggest that the underlying mechanisms whereby C/EBPβ deletion in hematopoietic-derived cells prevents atherosclerosis may be multifactorial and includes: a) improved mouse serum lipid profiles, specifically with lowered total and LDL cholesterol levels; b) reduced serum pro-inflammatory cytokine levels; and c) decreased macrophage-associated cholesterol accumulation and pro-inflammatory gene expression.
A surprising finding in the present study was a reduction in serum total and LDL cholesterol levels in C/EBPβ−/− → ApoE−/− HF/HC mice despite no change in HDL cholesterol. Our studies showed that C/EBPβ in the hematopoietic compartment modifies liver gene expression towards lowering cholesterol, including a dramatic increase in Lxr/Nr1h3 expression. Resident macrophages (i.e., Kupffer cells) are derived from hematopoietic stem cells and are primarily responsible for the removal of oxidized forms of LDL from plasma. Thus C/EBPβ deletion from bone marrow-derived cells, if infiltrated into the liver immune cell population, could play an important role in improved cholesterol balance in the liver [39]. Since the hematopoietic compartment serves as a reservoir for circulating monocytes and invading macrophages, the deletion of C/EBPβ in bone marrow may also play a protective role by transcriptional activation of Lxr/Nr1h3 and the downstream gene Cyp7a1, that contribute significantly to reducing lipids by increasing bile acid synthesis [40]. Lxr/Nr1h3 induction generally reduces hepatic cholesterol content by inducing reverse cholesterol transport, increasing bile acid production, and inhibiting intestinal cholesterol absorption, but does so at the expense of increases in lipogenesis resulting in hypertriglyceridemia and liver steatosis [41]. Notably, our results show that C/EBPβ deletion, specifically in the hematopoietic compartment, induces liver Lxr/Nr1h3 and reduces cholesterol without the added complication of liver steatosis. These data suggest that C/EBPβ deletion may hold promise for the treatment of lipid disorders, as well as preventing excess hepatic glucose production as shown previously in Leprdb/db mouse models [5].
The present study also demonstrated higher expression of Gpbar1/Tgr5 with bone marrow C/EBPβ deletion in ApoE−/− mice on HF/HC diet. The Gpbar1/Tgr5 receptor is highly expressed in hepatocytes and in macrophage cells as well as in other immune cells [42]. A well-defined function of Gpbar1/Tgr5 is its potent anti-inflammatory effect in the liver [43]. The activation of Gpbar1/Tgr5 in LDL−/− mice prevented atheroma development by reducing inflammation and lipid accumulation [44]. Thus the increased Gpbar1/Tgr5 found in the present study suggests that it may partly be responsible for the reduction of cholesterol and atherosclerosis development in C/EBPβ bone marrow-deficient ApoE−/− mice.
Because alterations in the lipoprotein profiles might contribute to the beneficial effect of C/EBPβ deletion on the aortic lesions in ApoE−/− mouse, we examined the direct effect of C/EBPβ knock-down in RAW264.7 macrophage cells in response to oxLDL-induced foam cell formation. Our study showed that deletion of C/EBPβ in RAW264.7 macrophage cells not only prevented macrophage foam cells but also attenuated inflammatory cytokine secretion in the medium. Therefore, it is likely that C/EBPβ deletion in macrophages (regardless of the source) may induce anti-atherogenic effects in atherosclerotic lesion by preventing lipid accumulation and pro-inflammatory cytokine production. Foam cell formation by cholesterol accumulation in arterial wall macrophages is a crucial event in the progression of atherogenesis that results from the deregulation of the balance between cholesterol influx and cholesterol efflux [37]. This balance depends on limiting cholesterol inflow through scavenger receptors, as well as maintaining outflow through reverse cholesterol transport, the transport of excess cholesterol from peripheral tissues, including cholesterol laden macrophages in vessel walls, to the liver for excretion [45,46]. Our results showing moderate increase in Abcg1 gene expression in C/EBPβ-depleted RAW macrophage cells suggests an increase in cholesterol efflux which may in part be responsible for the reduction in lipid accumulation in macrophages. However, the molecular signaling pathway(s) involved in C/EBPβ-mediated regulation of foam cell formation, macrophage recruitment, or tissue proliferation is not clear and studies are underway in our lab to clarify this point.
In addition to the novelty of our findings, our study is not without limitations. One of these is the small sample size (n = 4–5/group). While more mice would increase the power of some of the findings, we have clearly demonstrated a pronounced change of parameters associated with the progression of atherosclerosis in C/EBPβ−/− → ApoE−/− HF/HC mice compared with WT → ApoE−/− HF/HC mice and we have been conservative in our interpretation. The other limitation is that reconstitution of the ApoE−/− mice with WT bone marrow cells may compensate for the absence of ApoE and therefore can attenuate the complications and pathogenesis of atherosclerosis in ApoE−/− mice [47]. Future studies should include a C/EBPβ−/−/ApoE−/− double knockout mouse to further clarify C/EBPβ's mechanism in the regulation of atherosclerosis. Further, because RAW246.7 cells may not completely mimic tissue macrophages, these results bear repeating with isolated tissue macrophages from these mice.
In summary, C/EBPβ deletion, specifically in bone marrow-derived cells, reduced atherogenic lipid levels, total and LDL cholesterol in serum, suppressed inflammatory cytokines in the serum and significantly decreased atherosclerotic lesion formation in the aortic sinus of ApoE−/− mice, despite no differences in body weight. In vitro studies reveal that shRNA-mediated deletion of C/EBPβ in RAW macrophage cells attenuated oxLDL-mediated induction of foam cell formation, along with a moderate increase in Abcg1 gene expression and reduction in secretion of inflammatory cytokines in medium. Conservatively, these results indicate that C/EBPβ might be a crucial regulator of diet-induced inflammation, hyperlipidemia, and atherosclerosis. Thus inhibition of C/EBPβ might be an important therapeutic strategy for preventing atherosclerosis. Further studies in the time course and molecular events triggered by C/EBPβ in the macrophage appear warranted.
Supplementary Material
Acknowledgments
This study was supported by Beginning Grant in aid from the American Heart Association (09BGIA2060705, Southwest Affiliate) and start up funds from Texas Tech University to S.M.R., NIH grants R01-DK059767 to J.E.F. and P30DK048520 to the Colorado Nutrition and Obesity Research Center, and American Heart Association Beginning Grant in Aid (10BGIA458005 and 12 BGIA11380005, Southwest Affiliate) to M.M.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.atherosclerosis.2016.03.040.
References
- 1.Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu. Rev. Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.de Villiers WJ, Smart EJ. Macrophage scavenger receptors and foam cell formation. J. Leukoc. Biol. 1999;66:740–746. doi: 10.1002/jlb.66.5.740. [DOI] [PubMed] [Google Scholar]
- 4.Li P, Spann NJ, Kaikkonen MU, Lu M, Oh da Y, et al. NCoR repression of LXRs restricts macrophage biosynthesis of insulin-sensitizing omega 3 fatty acids. Cell. 2013;155:200–214. doi: 10.1016/j.cell.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroeder-Gloeckler JM, Rahman SM, Janssen RC, Qiao L, Shao J, et al. CCAAT/enhancer-binding protein beta deletion reduces adiposity, hepatic steatosis, and diabetes in Lepr(db/db) mice. J. Biol. Chem. 2007;282:15717–15729. doi: 10.1074/jbc.M701329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahman SM, Schroeder-Gloeckler JM, Janssen RC, Jiang H, Qadri I, et al. CCAAT/enhancing binding protein beta deletion in mice attenuates inflammation, endoplasmic reticulum stress, and lipid accumulation in diet-induced nonalcoholic steatohepatitis. Hepatology. 2007;45:1108–1117. doi: 10.1002/hep.21614. [DOI] [PubMed] [Google Scholar]
- 7.Zaret KS, Carroll JS. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 2011;25:2227–2241. doi: 10.1101/gad.176826.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem. J. 2002;365:561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poli V. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J. Biol. Chem. 1998;273:29279–29282. doi: 10.1074/jbc.273.45.29279. [DOI] [PubMed] [Google Scholar]
- 10.Cain DW, O'Koren EG, Kan MJ, Womble M, Sempowski GD, et al. Identification of a tissue-specific, C/EBPbeta-dependent pathway of differentiation for murine peritoneal macrophages. J. Immunol. 2013;191:4665–4675. doi: 10.4049/jimmunol.1300581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahman SM, Janssen RC, Choudhury M, Baquero KC, Aikens RM, et al. CCAAT/enhancer binding protein beta (C/EBPbeta) expression regulates dietary-induced inflammation in macrophages and adipose tissue in mice. J. Biol. Chem. 2012;287:34349–34360. doi: 10.1074/jbc.M112.410613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddick RL, Zhang SH, Maeda N. Atherosclerosis in mice lacking apo E. Evaluation of lesional development and progression. Arterioscler. Thromb. 1994;14:141–147. doi: 10.1161/01.atv.14.1.141. [DOI] [PubMed] [Google Scholar]
- 13.Kim DH, Sartor MA, Bain JR, Sandoval D, Stevens RD, et al. Rapid and weight-independent improvement of glucose tolerance induced by a peptide designed to elicit apoptosis in adipose tissue endothelium. Diabetes. 2012;61:2299–2310. doi: 10.2337/db11-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan L, Wang Y, Lu C, Li X. Angiotensin-converting enzyme 2 deficiency aggravates glucose intolerance via impairment of islet microvascular density in mice with high-fat diet. J. Diabetes Res. 2013;2013:405284. doi: 10.1155/2013/405284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 16.Matthan NR, Giovanni A, Schaefer EJ, Brown BG, Lichtenstein AH. Impact of simvastatin, niacin, and/or antioxidants on cholesterol metabolism in CAD patients with low HDL. J. Lipid Res. 2003;44:800–806. doi: 10.1194/jlr.M200439-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Kratzer A, Buchebner M, Pfeifer T, Becker TM, Uray G, et al. Synthetic LXR agonist attenuates plaque formation in apoE−/− mice without inducing liver steatosis and hypertriglyceridemia. J. Lipid Res. 2009;50:312–326. doi: 10.1194/jlr.M800376-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 20.Edsfeldt A, Grufman H, Asciutto G, Nitulescu M, Persson A, et al. Circulating cytokines reflect the expression of pro-inflammatory cytokines in atherosclerotic plaques. Atherosclerosis. 2015;241:443–449. doi: 10.1016/j.atherosclerosis.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Tie C, Gao K, Zhang N, Zhang S, Shen J, et al. Ezetimibe attenuates atherosclerosis associated with lipid reduction and inflammation inhibition. PLoS One. 2015;10:e0142430. doi: 10.1371/journal.pone.0142430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexopoulos N, Katritsis D, Raggi P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis. 2014;233:104–112. doi: 10.1016/j.atherosclerosis.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 23.Sui Y, Park SH, Xu J, Monette S, Helsley RN, et al. IKKbeta links vascular inflammation to obesity and atherosclerosis. J. Exp. Med. 2014;211:869–886. doi: 10.1084/jem.20131281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill AA, Reid Bolus W, Hasty AH. A decade of progress in adipose tissue macrophage biology. Immunol. Rev. 2014;262:134–152. doi: 10.1111/imr.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grundy SM. Hypertriglyceridemia, atherogenic dyslipidemia, and the metabolic syndrome. Am. J. Cardiol. 1998;81:18B–25B. doi: 10.1016/s0002-9149(98)00033-2. [DOI] [PubMed] [Google Scholar]
- 27.Weingartner O, Lutjohann D, Bohm M, Laufs U. Relationship between cholesterol synthesis and intestinal absorption is associated with cardiovascular risk. Atherosclerosis. 2010;210:362–365. doi: 10.1016/j.atherosclerosis.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Assini JM, Mulvihill EE, Sutherland BG, Telford DE, Sawyez CG, et al. Naringenin prevents cholesterol-induced systemic inflammation, metabolic dysregulation, and atherosclerosis in Ldlr(−)/(−) mice. J. Lipid Res. 2013;54:711–724. doi: 10.1194/jlr.M032631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doddapattar P, Radović B, Patankar JV, Obrowsky S, Jandl K, et al. Xanthohumol ameliorates atherosclerotic plaque formation, hypercholesterolemia, and hepatic steatosis in ApoE-deficient mice. Mol. Nutr. Food Res. 2013;57:1718–1728. doi: 10.1002/mnfr.201200794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiorucci S, Mencarelli A, Palladino G, Cipriani S. Bile-acid-activated receptors: targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol. Sci. 2009;30:570–580. doi: 10.1016/j.tips.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 2008;7:678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 32.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 34.Li AC, Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nat. Med. 2002;8:1235–1242. doi: 10.1038/nm1102-1235. [DOI] [PubMed] [Google Scholar]
- 35.Baldan A, Tarr P, Lee R, Edwards PA. ATP-binding cassette transporter G1 and lipid homeostasis. Curr. Opin. Lipidol. 2006;17:227–232. doi: 10.1097/01.mol.0000226113.89812.bb. [DOI] [PubMed] [Google Scholar]
- 36.Westerterp M, Bochem AE, Yvan-Charvet L, Murphy AJ, Wang N, et al. ATP-binding cassette transporters, atherosclerosis, and inflammation. Circ. Res. 2014;114:157–170. doi: 10.1161/CIRCRESAHA.114.300738. [DOI] [PubMed] [Google Scholar]
- 37.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol. Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 39.Bradshaw G, Gutierrez A, Miyake JH, Davis KR, Li AC, et al. Facilitated replacement of Kupffer cells expressing a paraoxonase-1 transgene is essential for ameliorating atherosclerosis in mice. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11029–11034. doi: 10.1073/pnas.0502677102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao C, Dahlman-Wright K. Liver X receptor in cholesterol metabolism. J. Endocrinol. 2010;204:233–240. doi: 10.1677/JOE-09-0271. [DOI] [PubMed] [Google Scholar]
- 41.Fievet C, Staels B. Liver X receptor modulators: effects on lipid metabolism and potential use in the treatment of atherosclerosis. Biochem. Pharmacol. 2009;77:1316–1327. doi: 10.1016/j.bcp.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 42.Keitel V, Haussinger D. Perspective: TGR5 (Gpbar-1) in liver physiology and disease. Clin. Res. Hepatol. Gastroenterol. 2012;36:412–419. doi: 10.1016/j.clinre.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Wang YD, Chen WD, Yu D, Forman BM, Huang W. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor kappa light-chain enhancer of activated B cells (NF-kappaB) in mice. Hepatology. 2011;54:1421–1432. doi: 10.1002/hep.24525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pols TW, Nomura M, Harach T, Lo Sasso G, Oosterveer MH, et al. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 2011;14:747–757. doi: 10.1016/j.cmet.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stephen SL, Freestone K, Dunn S, Twigg MW, Homer-Vanniasinkam S, et al. Scavenger receptors and their potential as therapeutic targets in the treatment of cardiovascular disease. Int. J. Hypertens. 2010;2010:646929. doi: 10.4061/2010/646929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Im SS, Osborne TF. Liver x receptors in atherosclerosis and inflammation. Circ. Res. 2011;108:996–1001. doi: 10.1161/CIRCRESAHA.110.226878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Eck M, Herijgers N, Yates J, Pearce NJ, Hoogerbrugge PM, et al. Bone marrow transplantation in apolipoprotein E-deficient mice. Effect of ApoE gene dosage on serum lipid concentrations, (beta) VLDL catabolism, and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 1997;17:3117–3126. doi: 10.1161/01.atv.17.11.3117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.