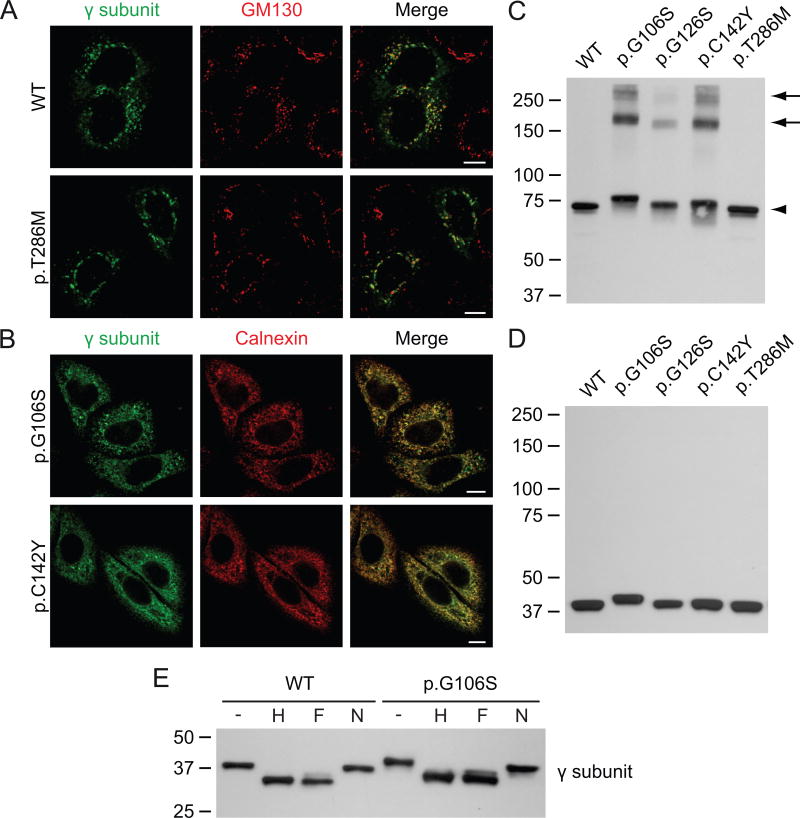
Figure 1. The γ subunit variants p.G106S, p.G126S and p.C142Y form aggregates in the ER.
A-B. Confocal immunofluorescence miscroscopy of HeLa cells transiently transfected with the WT αβ subunits of GlcNAc-1-phosphotransferase (not shown) and WT or variant γ subunit (anti-γ, green). WT and p.T286M γ co-localize with the cis-Golgi marker GM130 (red), 48h after transfection. The variants p.G106S and p.C142Y γ are retained in the ER, as shown by co-localization with calnexin (red). Scale bars, 10 μm. C-D. Western blot analysis (anti-FLAG antibody, showing the γ subunit) of transfected HEK293 cell lysates transiently transfected with the various γ subunit variants and the WT αβ subunits (not shown). The lysates were run in the absence (C) or the presence of reducing reagent (D). Note that the p.G106S γ variant migrates slower than the WT γ subunit. All variants formed dimers (C, arrowhead), while the variants p.G106S, p.G126S and p.C142Y also formed higher molecular weight aggregates (arrows). E. Western blots (anti-FLAG antibody) of the cell lysates from C treated for 3h at 37°C with Endo Hf (H) or PNGase F (F) show a similar shift as compared to the untreated lysates (-), indicating the presence of high mannose-type N-linked glycans. Neuraminidase (N) treatment had no apparent effect. The p.G106S variant behaved similar to WT, suggesting that the slower migration was not due to altered glycosylation.