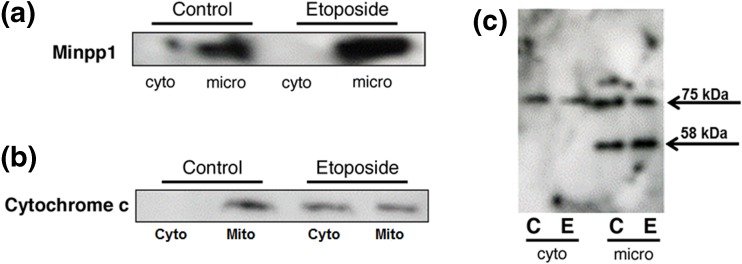
Fig. 2.
Subcellular localization and redistribution of proteins during apoptosis. Apoptosis in MC3T3-E1 cells was induced by incubating with 100 μM etoposide for 24 h followed by subcellular fractionation into microsomal (micro), cytosolic (cyto), and mitochondrial fractions. Equal amounts of proteins (50 μg) of various subcellular fractions separated by SDS-PAGE followed by western blotting as described in the “Materials and methods” section. a Immunoreactive bands were identified using anti-Minpp1 antibodies. Results show that Minpp1 expression was increased in etoposide-treated cells, but it was retained within microsomal fraction suggesting no redistribution to other compartments. Shown is a representative immunoblot out of three independent experiments with similar results. b Immunoreactive bands were identified using anti-cytochrome c antibody. Results show that etoposide treatment led to changes in mitochondrial membrane function and leakage of cytochrome c to the cytosol. Shown is a representative immunoblot out of three independent experiments with similar results. c Immunoreactive bands were identified using anti-KDEL antibody known to detect ER luminal proteins. This antibody detected only two bands (75 and 58 kDa) in MC3T3-E1 cells. Results show that the 75 kDa protein was distributed in both cytosolic and microsomal fractions, but the 58 kDa protein was present only in microsomal fraction, the expression of which was increased by etoposide treatment (C, control; E, etoposide). Shown is a representative immunoblot out of three independent experiments with similar results