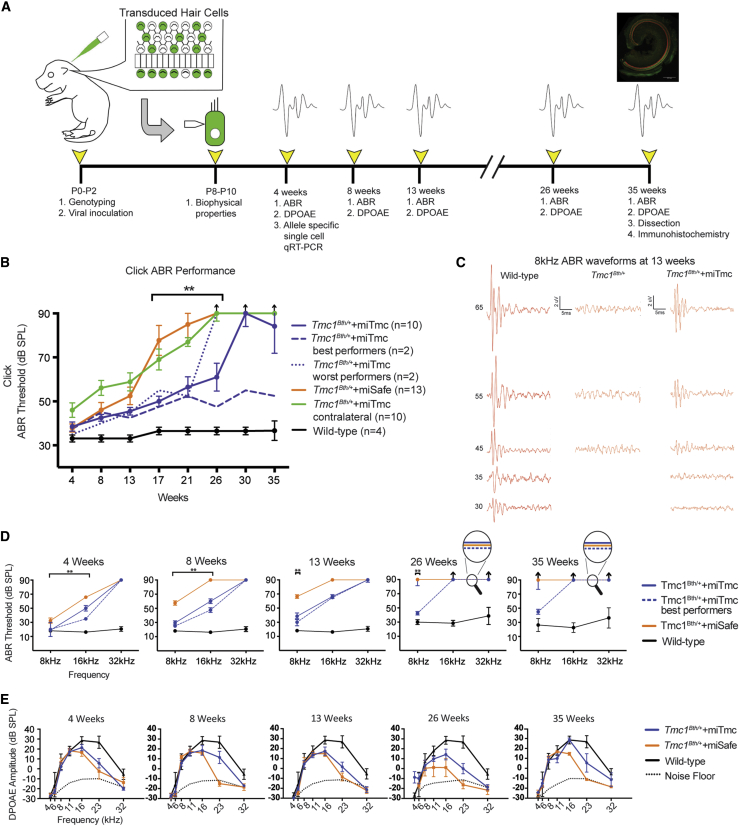
Figure 5.
miTmc Gene Therapy Slows Progression of Hearing Loss in Tmc1Bth/+ Mice
(A) Experimental timeline catalogs the experimental procedures in Tmc1Bth/+ mice and controls from the time of artificial miRNA injection to the time of tissue collection.
(B) Click ABR thresholds in wild-type, Tmc1Bth/++miTmc contralateral, Tmc1Bth/++miSafe, and Tmc1Bth/++miTmc animals. The two best-performing and two worst-performing Tmc1Bth/++miTmc-treated animals are shown as dashed and dotted blue lines, respectively, to illustrate variability in performance within the treated cohort.
(C) Representative 8 kHz ABR traces recorded from the wild-type, non-injected Tmc1Bth/++miTmc contralateral, and Tmc1Bth/++miTmc 13-week-old mice.
(D and E) Tone-burst ABR thresholds (D) and DPOAE amplitudes and noise floors (E) in wild-type, Tmc1Bth/+, Tmc1Bth/++miSafe, and Tmc1Bth/++miTmc animals at 4, 8, 13, 26, and 35 weeks. The dotted black line indicates the average noise floor for each group of DPOAEs. Black arrows indicate no response at equipment limits. ∗p < 0.05, ∗∗p < 0.005. See also Figures S4 and S5.