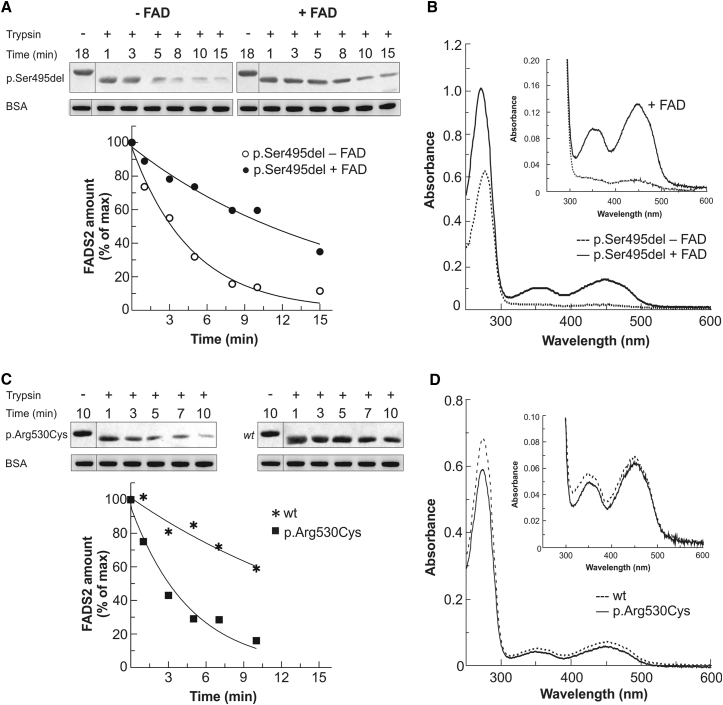
Figure 4.
Characterization of Recombinant 6His-p.Arg530Cys and 6His-p.Ser495del FADS2 Proteins
(A and B) The trypsin sensitivity (A) and degree of FAD saturation (B) of 6His-p.Ser495del FADS2 are reported in comparison with those of the same protein after reconstitution with a 2.5-fold molar excess of FAD. In (B), spectra of both 6His-p.Ser495del FADS2 purified in its apoform (0.58 mg/mL protein concentration, dashed line) and the reconstituted holoform (0.64 mg/mL protein concentration, black line) are reported.
(C and D) The trypsin sensitivity (C) and degree of FAD saturation (D) of 6His-p.Arg530Cys FADS2 are reported in comparison with those of WT FADS2. In (D), spectra of both 6His-p.Arg530Cys FADS2 (0.44 mg/mL protein concentration, straight line) and WT protein (0.5 mg/mL protein concentration, dashed line) are reported.
Trypsin sensitivity was analyzed by immunoblotting. The control represents protein treated under the same condition but in the absence of trypsin (−). The slower migration of the control band might reflect that the 6His tag is rapidly removed upon addition of trypsin. BSA was added to the loading buffer as an internal standard. In the graph, the time course of the limited proteolysis is reported as a percentage of the control amount (arbitrarily set to 100%). The degree of FAD saturation was estimated from the UV/Vis absorbance spectra.