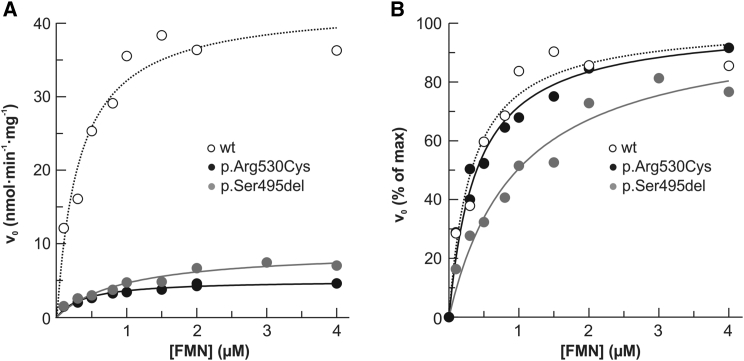
Figure 5.
Kinetic Characterization of Recombinant 6His-p.Arg530Cys and 6His-p.Ser495del FADS2 Proteins
The dependence of FMN concentration on the rate of FAD synthesis catalyzed by WT (0.17 nmol), 6His-Arg530Cys (0.17 nmol), or 6His-Ser495del (0.18 nmol) FADS2. The FAD synthesis catalyzed by WT FADS2 is presented as open symbols. Mutant FADS is presented as closed symbols. The rate of FAD synthesis was measured in the presence of 100 μmol/L ATP and 5 mmol/L MgCl2. v0 was measured by the initial rate of fluorescence decrease (excitation at 450 nm and emission at 520 nm) and expressed in nmol FMN · min−1 · mg−1 mutant FADS2 (A) and as a percentage of the Vmax value (set arbitrarily to 100%) (B). Data points are fitted according to the Michaelis-Menten equation.