Figure 1.
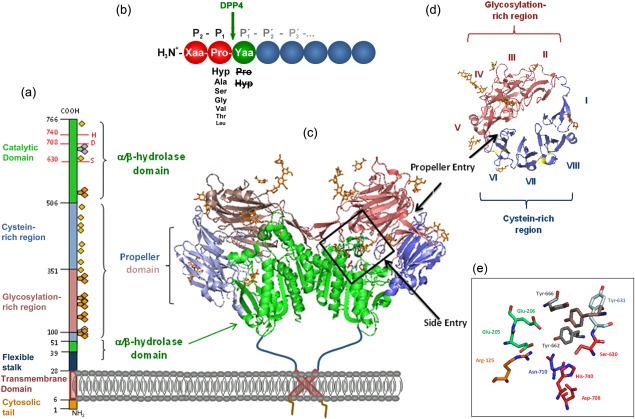
Primary and quaternary structure of human dipeptidyl peptidase 4 (DPP4), based on Protein Data Bank: 1W1I. (a) Primary structure of DPP4 subunit, consisting of an intracellular tail (aa 1–6), transmembrane region (aa 7–28), flexible stalk (aa 29–39), glycosylated region (aa 101–350), cysteine‐rich region (aa 55–100, 351–497), and catalytic region (aa 506–766).  , N‐glycosylation;
, N‐glycosylation;  , potential unoccupied N‐glycosylation;
, potential unoccupied N‐glycosylation;  , cysteine residues involved in S‐bridges; red numbers and letters indicate the catalytic triad. (b) Substrate specificity of DPP4. Xaa and Yaa indicate any amino acid. Decreasing font of amino acid at P1 position represents declining rate of hydrolysis. Amino acids crossed out must not occupy P1′. Arrow indicates site of cleavage. (c) quaternary structure of homodimeric human recombinant DPP4 as determined by Weihofen et al., 2004, showing the α/β‐hydrolase domain (aa 39–51 and 506–766) in green and propeller domain (aa 55–497) with the glycosylation‐rich subdomain (red) and the cystein‐rich subdomain (blue). (d) Propeller domain viewed from the top, illustrating the eight propeller blades designated with roman numbers and two subdomains. (e) Active site zoomed in, depicting the residues involved in catalysis, catalytic triad Ser630, Asp708, His740 are shown in red, Tyr547 responsible for oxyanion hole in brown, Tyr662 and Tyr666 forming the hydrophobic pocket in grey, Arg125 and Asn710, contributing to an electrostatic sink in orange and blue, respectively, and Glu205 and Glu206 ensuring N‐terminal anchoring in pale green. S–S bridges are illustrated in yellow and carbohydrates in orange. Structures were drawn with PyMOLTM 2008 DeLano Scientific LLC, using Protein Data Base: 1W1I 7.
, cysteine residues involved in S‐bridges; red numbers and letters indicate the catalytic triad. (b) Substrate specificity of DPP4. Xaa and Yaa indicate any amino acid. Decreasing font of amino acid at P1 position represents declining rate of hydrolysis. Amino acids crossed out must not occupy P1′. Arrow indicates site of cleavage. (c) quaternary structure of homodimeric human recombinant DPP4 as determined by Weihofen et al., 2004, showing the α/β‐hydrolase domain (aa 39–51 and 506–766) in green and propeller domain (aa 55–497) with the glycosylation‐rich subdomain (red) and the cystein‐rich subdomain (blue). (d) Propeller domain viewed from the top, illustrating the eight propeller blades designated with roman numbers and two subdomains. (e) Active site zoomed in, depicting the residues involved in catalysis, catalytic triad Ser630, Asp708, His740 are shown in red, Tyr547 responsible for oxyanion hole in brown, Tyr662 and Tyr666 forming the hydrophobic pocket in grey, Arg125 and Asn710, contributing to an electrostatic sink in orange and blue, respectively, and Glu205 and Glu206 ensuring N‐terminal anchoring in pale green. S–S bridges are illustrated in yellow and carbohydrates in orange. Structures were drawn with PyMOLTM 2008 DeLano Scientific LLC, using Protein Data Base: 1W1I 7.
