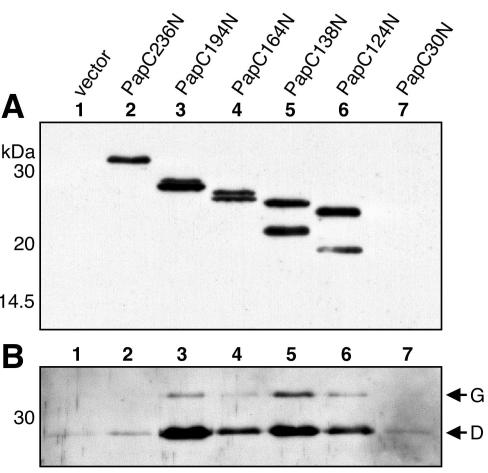
FIG. 8.
Copurification of chaperone-adhesin complexes with the PapC N-terminal fragments. Periplasm was isolated from SF100 expressing the vector alone or the indicated PapC N-terminal fragment, and the fragments were then purified by their His tags with nickel beads. Duplicate samples were subjected to SDS-PAGE and transferred to PVDF membrane. (A) The amount of N-terminal fragments purified was detected by blotting with anti-His tag antibody. Protein was recovered for each of the fragments except PapC30N. (B) PapDG chaperone-adhesin complexes that were copurified with the N-terminal fragments were detected by blotting with anti-PapDG antibody. PapDG was copurified with PapC fragments containing 124, 138, 164, and 194 residues of the mature N terminus.