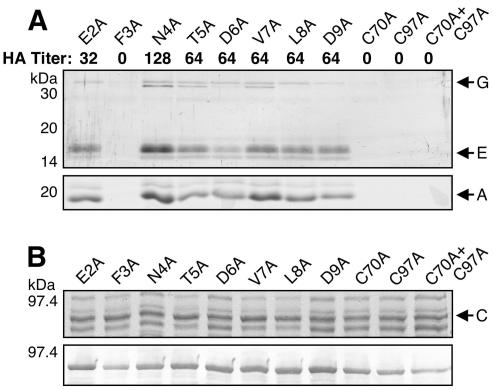
FIG. 9.
(A) Analysis of pilus biogenesis by the alanine substitution PapC mutant proteins. AAEC185/pMJ2 (ΔpapC pap operon) was complemented with the indicated PapC mutant protein, and assembly of adhesive pili was measured by agglutination of human erythrocytes. The HA titer is the highest fold dilution of bacteria that still provides agglutination. The HA titer of WT PapC was 128. Assembly of pili on the bacterial surface was determined by heat extraction and magnesium precipitation, followed by SDS-PAGE. The rod subunit PapA was detected by Coomassie blue staining (bottom). The tip fibril subunits PapE and PapG were detected by blotting with anti-P pilus tip antibody (top). The F3A, C70A, C97A, and C70A+C97A PapC mutant proteins were nonfunctional for pilus biogenesis and agglutination. These samples were analyzed together with the WT PapC and vector controls shown in Fig. 3A. (B) Overlay assay for targeting of PapDG chaperone-adhesin complexes to the alanine substitution PapC mutant proteins. The OM was isolated from SF100 expressing the indicated PapC mutant protein. Duplicate samples were separated by SDS-PAGE and either stained with Coomassie blue to show the amount of PapC loaded (top) or transferred to PVDF membrane for the overlay assay (bottom). The PVDF membrane was incubated with PapDG-containing periplasm, and PapDG binding to PapC was detected by blotting with anti-PapDG antibody. All of the PapC mutant proteins were able to bind PapDG complexes. These samples were analyzed together with the WT PapC and vector controls shown in Fig. 3B.