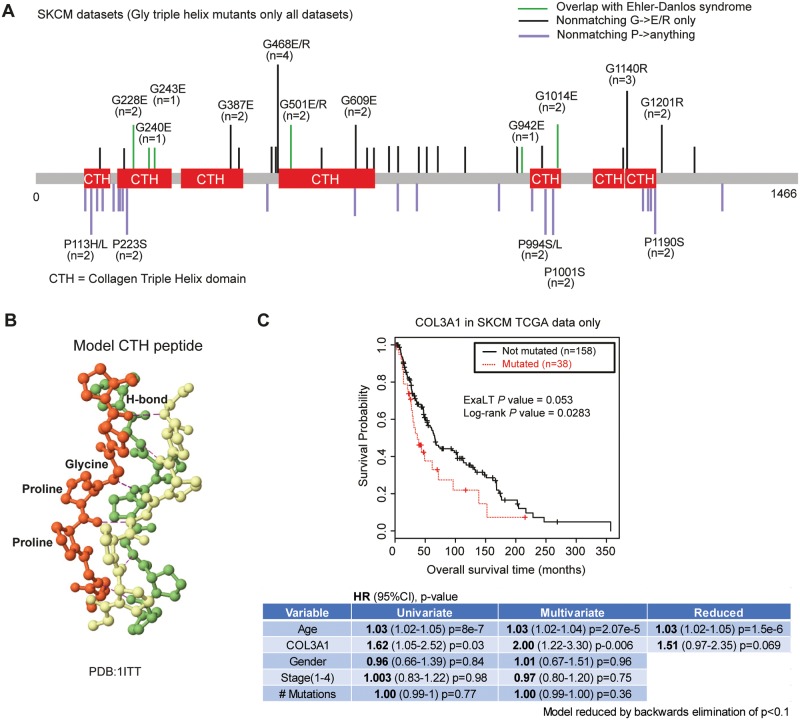
Fig 4. COL3A1 mutations are associated with melanoma.
(A) Reported mutations in COL3A1 for all skin cutaneous melanoma studies. Mutations overlapping with Ehlers-Danlos syndrome are shown in green. Only glycine -> charged residue mutations (E/R) in conserved regions are shown for clarity. Examination of all melanoma mutations in COL3A1 revealed numerous other glycine triple helix mutations that were distributed along the length of the protein, but are not documented to cause Ehlers-Danlos syndrome in the HUMSAVAR database (shown in black and labeled if N>2). Proline residues that also are important for the triple helix were also mutated (shown below in purple). (B) A model of collagen triple helix structure is shown. Hydrogen bonds stabilizing the triple helix conformation are shown. The tight-steric interactions of the proline residues and hydrogen bonding between the backbone nitrogen of the glycine residue and a carbonyl on an adjacent strand stabilize the triple helix conformation. Substitution of glycine with a bulkier residue directly disrupts these interactions. C) A Kaplan—Meier curve for all COL3A1 mutations in the TCGA (others non-TCGA studies did not have survival data). COL3A1 in SKCM was found to be modestly associated with decreased overall survival based upon log-rank, ExaLT and/or Cox regression. Cox regression outputs for univariate, multivariate, and reduced models with different effect sizes and P values are shown below the Kaplan-Meier curve. SKCM, skin cutaneous melanoma.