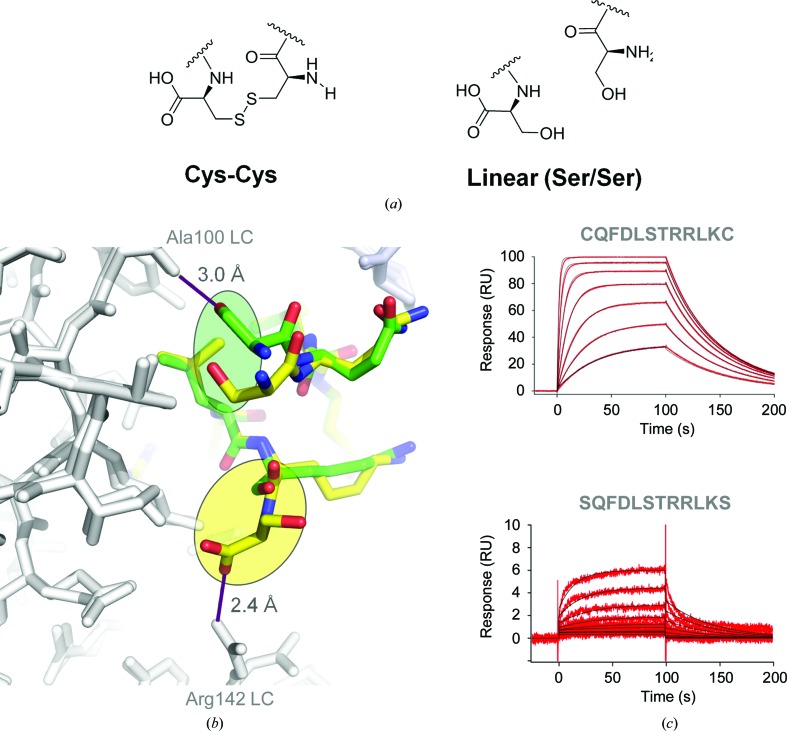
Figure 2.
Cyclic versus acyclic. (a) Replacement of Cys1 and Cys12 with serine as a conservative substitution to create a linear meditope. (b) There are two Fab–peptide complexes in the asymmetric unit. Superposition of these complexes indicates flexibility at the N-termini. In one peptide Fab–complex (the peptide with yellow C atoms), the terminal carboxylate makes a favorable salt bridge with the Arg142 guanidinium group from the light chain (LC). In the other peptide–Fab complex (the peptide with green C atoms), the hydroxyl group of Ser1 makes a hydrogen bond to the carbonyl group of Ala100, also of the light chain. However, the electron density is weak and Ser12 could not be built. (c) SPR traces of the original meditope and the linear meditope indicate a substantial reduction in binding affinity and an increase in the off-rate.