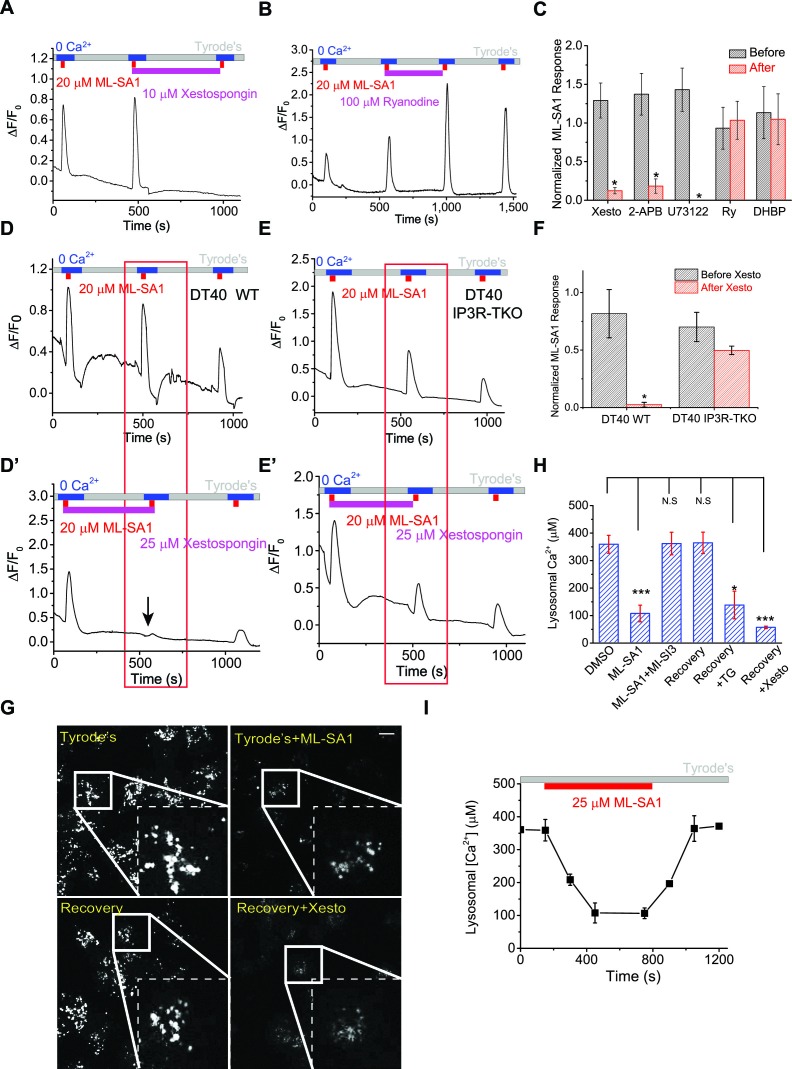
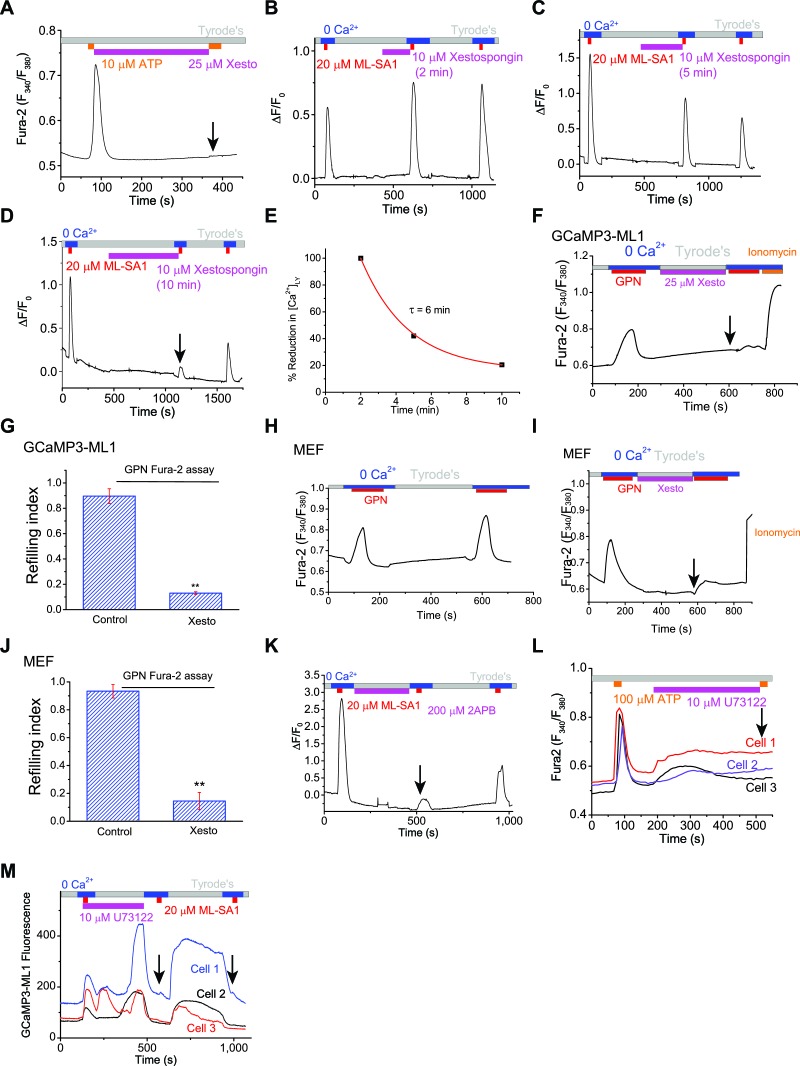
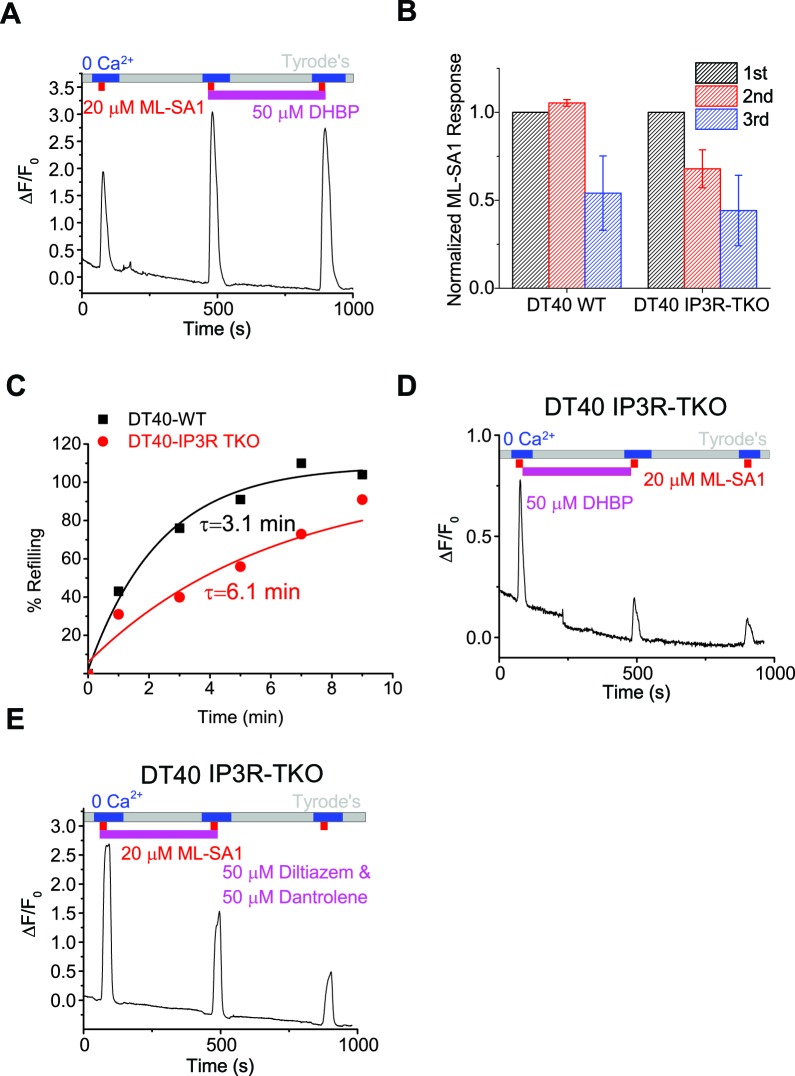
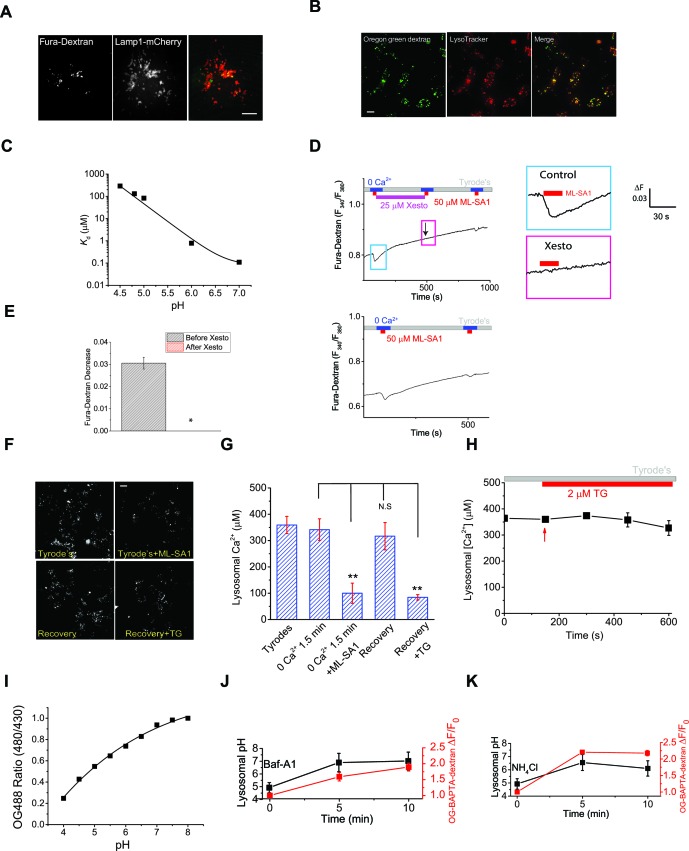
Figure 3. IP3-receptors on the ER are required for lysosomal Ca2+ store refilling.
(A) The IP3-receptor (IP3R) antagonist Xestospongin-C (Xesto, 10 μM) prevented Ca2+ refilling of lysosomes in HEK-GCaMP3-ML1 cells (p=0.007). Note that Xesto was co-applied with ML-SA1. (B) Ryanodine (100 μM), which blocks Ryanodine receptors at high concentrations, did not block Ca2+ refilling to lysosomes. Note that Ryanodine was co-applied with ML-SA1. (C) Quantification of the responses to ML-SA1 in HEK-GCaMP3-ML1 cells after treatment with Xesto, 2-APB (Figure 3—figure supplement 1K), U73122 (Figure 3—figure supplement 1L,M), Ryanodine (Ry), and DHBP (Figure 3—figure supplement 2A) (Figure 3—source data 1).(D) DT40 WT cells transiently transfected with GCaMP3-ML1 show Ca2+ refilling. (D’) IP3R antagonist Xesto completely blocked Ca2+ refilling of lysosomes in DT40 WT cells. (E) DT40 IP3R triple KO (TKO) cells transiently transfected with GCaMP3-ML1 also show Ca2+ refilling. (E’) Xesto did not block Ca2+ refilling of lysosomes in IP3R-TKO cells. (F) Quantification of ML-SA1 responses with or without Xesto in WT and IP3R-TKO DT40 cells (Figure 3—source data 1). (G) Representative images showing the effects of Xesto on the recovery of ML-SA1-induced responses in HEK-ML1 stable cells loaded with OG-BAPTA-dextran. La3+ was used to block external Ca2+ influx that could be mediated by surface-expressed ML1 in the overexpression system (see Figure 1—figure supplement 2G). (H) The effects of TG and Xesto on intralysosomal Ca2+ contents measured by OG-BAPTA-dextran (Figure 3—source data 1). (I) The effects of ML-SA1 on [Ca2+]Ly measured by OG-BAPTA-dextran. Panels A, B, D, D’, E, E’, F, F’ and H are the average of 30–40 cells from one representative experiment. The data in panels C, F and H represent mean ± SEM from at five independent experiments. The scale bar in panel G = 10 μm.
DOI: http://dx.doi.org/10.7554/eLife.15887.012