Figure 1.
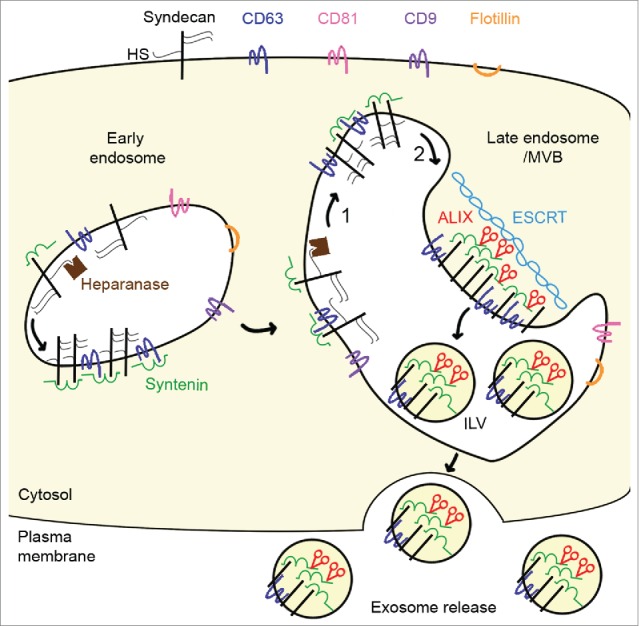
Heparanase tailors syndecan for exosome production. Syndecan and heparan sulfate (HS) cargo (not shown) are internalized. In endosomes, syntenin directly interacts with syndecans and the tetraspanin cluster of differentiation 63 (CD63) via its tandem postsynaptic density 95/disc-large/zona occludens (PDZ) domains. During endosome maturation into late endosomes, syndecans are trimmed by heparanase (1) and undergo proteolytic cleavage of their extracellular part (2) to generate a membrane-associated syndecan C-terminal fragment. These cleavages allow syndecan to cluster, and stimulate endosomal budding into intraluminal vesicles (ILV) and exosome release. The endosomal budding of syndecan-syntenin-CD63 also depends on the direct interaction of the N-terminal domain of syntenin with the endosomal-sorting complex required for transport (ESCRT) accessory component ALG-2-interacting protein X (ALIX), and on several ESCRT proteins. Heparanase does not stimulate all types of exosomes; it stimulates the release of syndecan C-terminal fragment, syntenin, ALIX and CD63, but has no effect on the release of exosomal flotillin, CD9, or CD81.
