Abstract
Other than hydroxyurea, no pharmacologic agents are clinically available for fetal hemoglobin (HbF) induction in sickle cell disease. An optimal candidate would induce HbF without causing cell cycle inhibition and would act independently of hydroxyurea in order to yield additional HbF induction when combined. We explored whether inhibition of histone deacetylase (HDAC) 1 or HDAC2 could achieve these goals. In human erythroid progenitor cells, shRNA knockdown of the HDAC1 or HDAC2 genes induced gamma globin, without altering cellular proliferation in vitro, and without altering cell cycle phase. Treatment with hydroxyurea in combination with HDAC2 knockdown yielded a further increase in gamma globin expression. Additionally, when CD34+ cells were treated with both hydroxyurea and MS-275 (an inhibitor of HDAC 1, 2, and 3), an additive induction of relative gamma globin expression was achieved. Our findings support further clinical investigation of HDAC inhibitors in combination with hydroxyurea in sickle cell disease patients.
Keywords: sickle cell disease, fetal hemoglobin, histone deacetylase (HDAC) 1, histone deacetylase (HDAC) 2
Introduction
Sickle cell disease (SCD) is one of the most common genetic diseases in the world. The prevalence of SCD is highest in sub-Saharan Africa, where more than 230,000 affected children are estimated to be born each year1. In North America, the yearly estimate of affected births is 26001, with approximately 70,000 to 100,000 individuals of all ages affected in the United States2. The clinical manifestations of SCD include acute events, such as recurrent debilitating painful crises, as well as life-threatening pulmonary, cardiovascular, renal, and neurologic complications. Unfortunately, SCD is a condition that has been primarily bypassed by the era of targeted molecular therapeutics, and the mainstay of management is still symptomatic control, which is not fully effective and often produces adverse effects.
Increasing fetal hemoglobin (HbF) has long been a therapeutic goal in sickle cell disease (SCD) because HbF is a potent inhibitor of the polymerization of deoxyhemoglobin S3. However, hydroxyurea remains the only HbF induction agent approved by the Food and Drug Administration for SCD patients. Hydroxyurea, an S-phase cytotoxic agent and ribonucleotide reductase inhibitor, was first shown to increase HbF in patients with sickle cell disease in 19844, and was FDA approved for this indication over 15 years ago in 19982. Hydroxyurea is a valuable medication and has been shown to effectively decrease painful crises, transfusion requirement, incidence of acute chest syndrome, hospitalization5, as well as mortality6–8 in adults, and to be safe and efficacious in children 2,9–11. However, hydroxyurea is not effective for every patient with SCD. For example, in a cohort of 53 pediatric patients from the HUG-KIDS trial, approximately 10% of the patients increased their fetal hemoglobin levels less than 2% from baseline.12 Even if fetal hemoglobin increases, some patients fail to experience a clinical improvement in symptoms and complications. Additionally, some patients develop adverse effects from HU, most commonly neutropenia, that precludes dose escalation to an effective dose.
The clinical development of novel, safe, and easily administered drugs or combinations that achieve the objective of HbF induction remains a critical endeavor. In addition to hydroxyurea,4 known activators of HbF include hypomethylating agents such as 5-azacytidine and decitabine,13–15 and histone deacetylase (HDAC) inhibitors, including short-chain fatty acid derivatives such as butyrate16–21. Unfortunately, despite promising preclinical potential, these agents could not be successfully translated to routine clinical use because of factors including short half-life and cumbersome route of administration. Recently, a trial of a novel agent HQK-1001, a short-chain fatty acid butyrate derivative, unfortunately showed no significant increase in HbF22. All of these molecules act at least in part via cell cycle inhibition, and myelosuppression is the primary dose-limiting toxicity of hydroxyurea. A molecule that induces HbF without cell cycle inhibition could potentially be used in combination with hydroxyurea to achieve increased HbF induction with non-overlapping toxicities.
By blocking the activity of histone deacetylase enzymes, HDAC inhibitors trigger hyperacetylation of ε-amino groups of lysine residues in histones. This causes a decreased association of basic core histone proteins with the DNA, causing some genes to be more available for transcription19. The HDAC class of enzymes includes multiple isoforms that have potentially selective biological roles, including in the regulation of HbF. Prior studies demonstrated that decreased expression of HDAC1 or HDAC2 is sufficient for induction of HbF.23 Non-selective HDAC inhibitors have been shown to block cell cycle progression via p21 induction in a variety of in vitro and in vivo cellular contexts.24,25 Indeed, non-selective HDAC inhibitors have been widely studied in experimental models designed to test their anti-proliferative effects for therapeutic efficacy in cancer (reviewed in New et al26). However, less is known about the role of specific HDAC enzymes or their inhibitors on the proliferation of non-malignant tissues. If a selective HDAC inhibitor could induce HbF without altering cell cycle, then treating SCD patients with a combination of hydroxyurea and selective HDAC inhibitors has the potential to achieve greater efficacy without increased toxicity. We therefore tested whether selective inhibition of individual HDAC enzymes can increase gamma globin expression without altering cell cycle, and whether combining HDAC inhibition with hydroxyurea achieves an additive effect on HbF induction.
Methods
Western blot analysis
Antibodies against HDAC1 (HDAC1 (10E2) mouse monoclonal antibody; Santa Cruz Biotechnology) and HADC2 (HDAC2 rabbit polyclonal antibody; Cell Signaling) were used at a 1:200 dilution. Beta-actin (C4) mouse monoclonal IgG1 (Santa Cruz Biotechnology) was used as a control at a 1:5000 dilution. The target proteins were analyzed using SuperSignal West Pico Chemiluminescent Substrate for horseradish peroxidase (ThermoScientific).
Culture of primary CD34+ cells and cDNA synthesis
Cryopreserved human bone marrow CD34+ cells were obtained from Poietics. Erythroid differentiation was induced in vitro in two steps as described previously23. For the first 7 days, cells were cultured in serum-free expansion medium (Stem Cell Technologies) supplemented with 100 U/mL penicillin/streptomycin, 2 mM glutamine, 100 ng/mL stem cell factor, 10 ng/mL interleukin-3, 40 µg/mL lipids, and 0.5 IU/mL erythropoietin. After 7 days, cells were cultured in the same medium supplemented with 3 IU/mL erythropoietin. The MultiMACS Separator/Column system (Miltenyi) was used to isolate mRNA and synthesize cDNA.
Lentiviral vectors and infection
Target sequences of shRNAs are listed in supplemental Table 1. The lentiviral backbone vector (pLKO.1) and packaging plasmids were transfected into 293T cells and the viral supernatant was harvested as described previously27. Cryopreserved, primary hematopoietic cells were infected with lentivirus 1 day after thawing in the presence of 2 µg/mL Polybrene (Sigma-Aldrich) and selected 24 hours later with 2 µg/mL puromycin (Sigma-Aldrich).
Real-time RT-PCR
TaqMan primers and probes for PCR were obtained from Applied Biosystems. Each quantitative RT-PCR was performed in triplicate using a Prism 7900 HT instrument (Applied Biosystems). The mean threshold cycle (Ct) for each assay was used for further calculations. The expression of γ and δ globin were normalized to β-globin (ΔCt). The expression of p21 was normalized to actin or GAPDH. The ΔΔCt value was calculated by normalizing the ΔCt value to a vehicle-treated control sample. The triplicates in all qPCR experiments were biological replicates, from multiple separate samples.
Compounds
Hydroxyurea (Sigma) was dissolved in water to make a fresh 10mM stock solution just prior to use in each experiment. MS-275 (Santa Cruz) was dissolved to make a 10mM stock solution in DMSO, and diluted in fresh media just prior to use in each experiment. DMSO was added in equivalent concentrations to control samples.
BrdU incorporation assay
Cells were treated with a 30-minute pulse of BrdU and were then stained according to the manufacturer’s instructions (BD Bioscience).
Results
Effect of HDAC1 or HDAC2 knockdown on proliferation and cell cycle
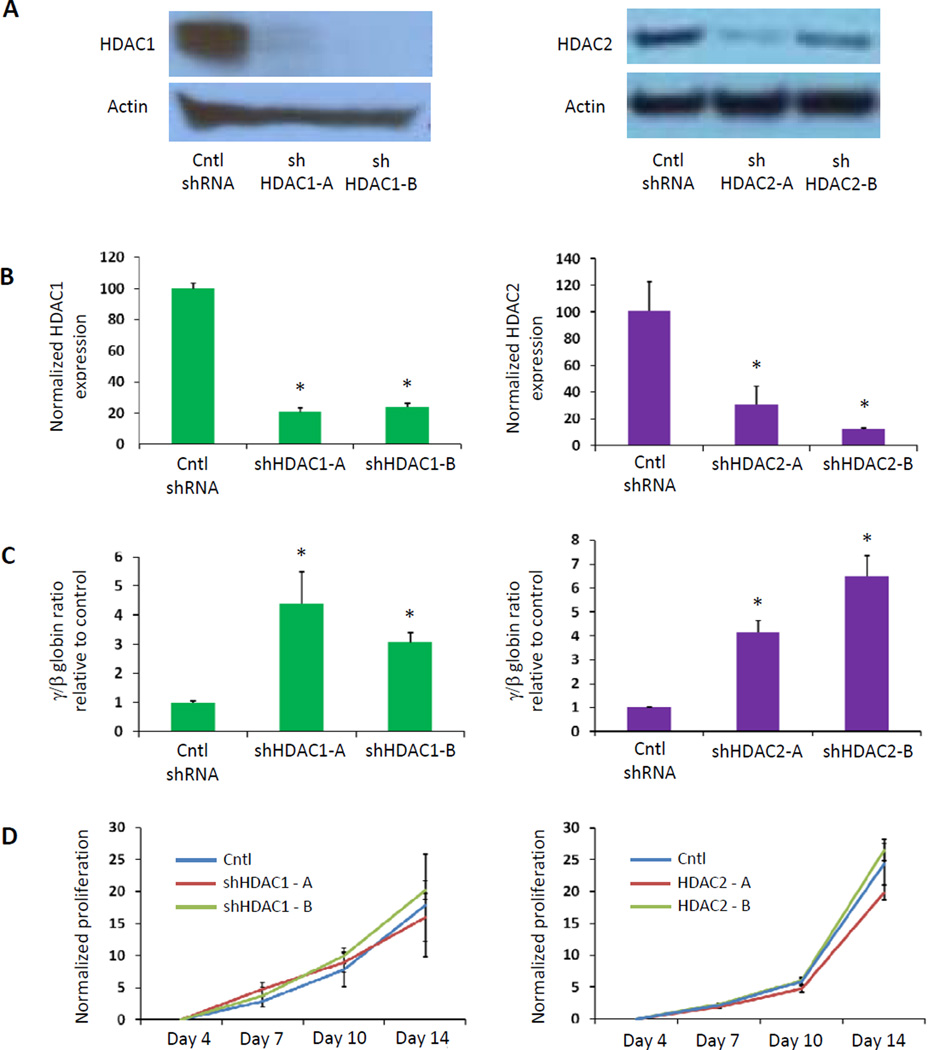
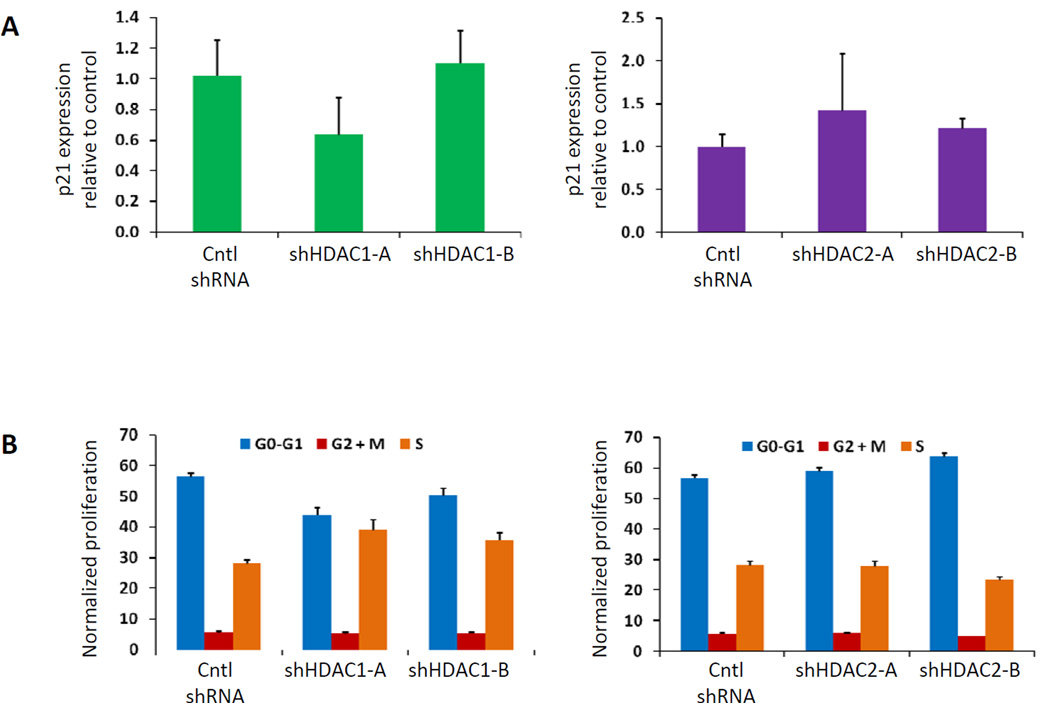
We sought to determine whether knockdown of HDAC1 or HDAC2 could induce HbF without altering cell cycle or proliferation in primary human bone marrow hematopoietic progenitor cells generated from the in vitro differentiation of human CD34+ bone marrow cells. We employed shRNAs against HDAC1 and HDAC2 that effectively decrease expression of the target mRNA (Figure 1B) and protein (Figure 1A). We confirmed that infection with lentiviruses containing these shRNAs caused induction of gamma globin expression (Figure 1C). Next we tested whether cellular proliferation was affected by quantifying cell number in culture for 14 days after lentiviral infection. As shown in Figure 1D, knockdown of HDAC1 or HDAC2 expression in human erythroid progenitor cells did not prevent cellular expansion. Because global HDAC inhibition has been associated with cell cycle inhibition via p21 induction24, we examined the effect of selective HDAC1 or HDAC2 knockdown on p21 expression. In contrast to the effect of pan-HDAC inhibition, we found no significant p21 induction after knockdown of HDAC1 or HDAC2 alone (Figure 2A). Finally, we found that the percent of cells in each cell cycle phase did not change significantly when HDAC1 or HDAC2 was knocked down (Figure 2B). These experiments demonstrate that selective inhibition of specific HDAC enzymes via shRNA knockdown can induce HbF without altering cell cycle.
Figure 1. Inactivation of HDAC1 or HDAC2 induces gamma globin expression without blocking cellular proliferation.
(A) A Western blot shows the decreased level of protein with HDAC1 knockdown (left panel) and with HDAC2 knockdown (right panel). (B) Lentiviruses expressing shRNAs targeting HDAC1 (1-A and 1-B) or HDAC2 (2-A and 2-B) effectively decreased expression of the target mRNA, and (C) increased expression of γ-globin relative to β-globin in primary human erythroid progenitor cells. (D) After infection with the shRNA-expressing lentiviruses, cells in culture were counted over the course of 14 days, and normalized to the number of cells 3 days after infection, following selection with puromycin. In panels B and C, a 2-tailed Student t test was used. *P ≤ .01.
Figure 2. Inactivation of HDAC1 or HDAC2 does not affect p21 expression or cell cycle phase.
(A) Level of p21 expression was assessed by qPCR in cells harvested three days after lentiviral infection. (B) Cell cycle was analyzed by flow cytometry. Following a 30 minute pulse of BrdU, cells were collected and fixed ten days after infection. After staining with an antibody against BrdU and 7-AAD, cells were analyzed by FACS. A representative flow plot is shown in Figure S1. In panel A, a 2-tailed Student t test was used, and samples were not statistically different from controls (P > .1).
Combination of HDAC inactivation and hydroxyurea
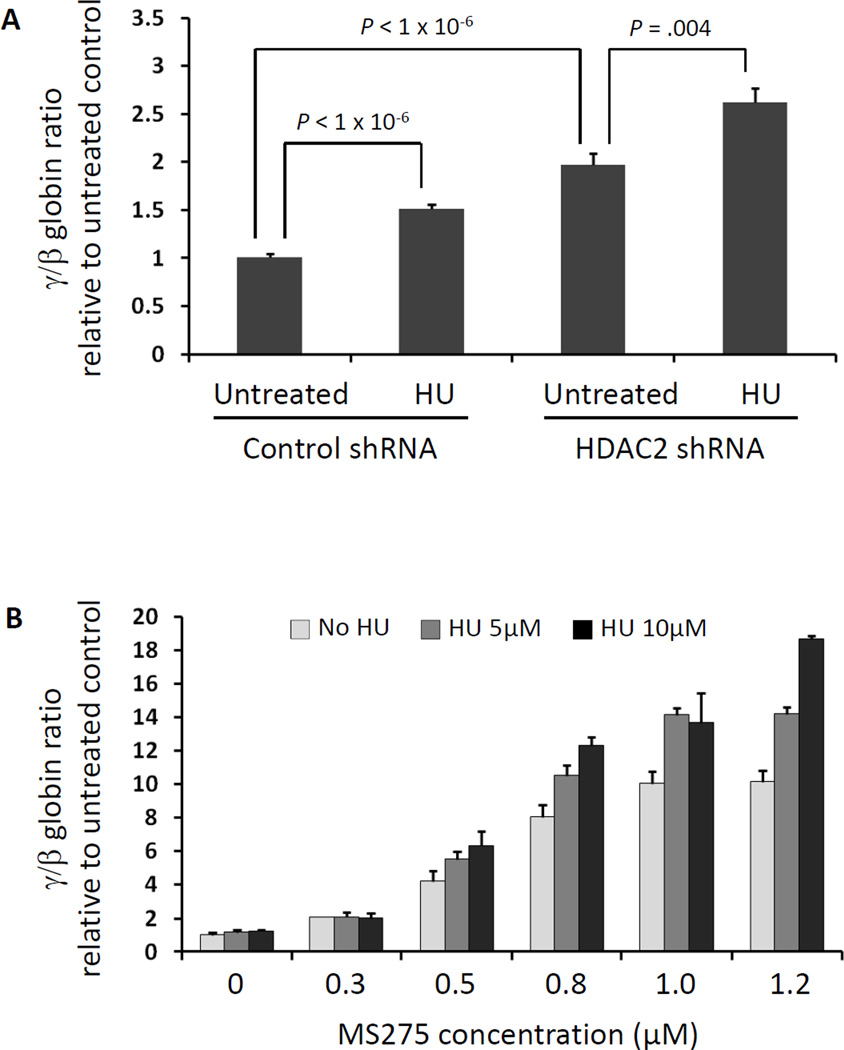
Hydroxyurea is an FDA-approved therapy for SCD that acts in part as a ribonucleotide reductase inhibitor, causing inhibition of the cell cycle inhibitor, and induces HbF. Having demonstrated that knockdown of specific HDAC enzymes increases gamma globin expression without altering cell cycle status, we tested whether HDAC inhibitors and hydroxyurea might have additive effects. Combining HDAC2 knockdown with hydroxyurea treatment yielded a further increase in gamma globin induction (Figure 3A). These results indicate that hydroxyurea and HDAC inhibitors act through independent mechanisms and have additive activity. To investigate the potential efficacy of combination therapy, we treated CD34+ cells with combinations of hydroxyurea and MS-275. MS-275, also known as entinostat, is a benzamide HDAC inhibitor that targets HDACs 1, 2, and 323, and it is currently in use in multiple clinical trials for a variety of malignancies. We found that simultaneous treatment with both drugs was additive for the relative induction of gamma globin expression, as shown in Figure 3B, further suggesting that hydroxyurea and MS-275 have independent mechanisms and additive efficacy.
Figure 3. HDAC inactivation in combination with hydroxyurea has additive effects on γ-globin expression.
(A) Primary human erythroid progenitor cells were infected with lentiviruses expressing shRNAs targeting HDAC2. Each population of infected cells was treated with either hydroxyurea (HU) or vehicle control. Expression of γ-globin relative to β-globin, measured by qPCR, was the highest in cells with both HDAC2 knockdown and HU treatment. (B) Primary human erythroid progenitor cells were treated with hydroxyurea, MS-275, or a combination of both, at multiple doses. At each dose of MS-275, the γ-globin induction is further increased by the addition of hydroxyurea, in a dose-dependent fashion.
Discussion
Despite the prevalence of SCD, the well-established pathophysiology of the disorder, and the validation of HbF induction as a therapeutic approach, few clinical trials of novel agents have been performed in SCD. Hydroxyurea is still not approved for children with sickle cell disease, and even among adults, it is only approved for those who have pre-defined severe disease manifested by frequent vaso-occlusive events. As demonstrated by decreased quality of life28,29, missed school and work, and healthcare costs, sickle cell disease remains an incredible burden on patients and their families, as well as the societies in which it exists. The need for novel treatment options is great.
Hypomethylating agents and short chain fatty acid derivatives that also have low-potency, non-selective HDAC inhibitor activity, all of which have cell cycle inhibitory activity, have a strong clinical trial history in SCD, including the use of 5-azacytadine (cite 14), decitabine (cite 15), and butyrate (cite 16–18),. Unfortunately, despite in vitro efficacy, these agents could not be clinically adopted because of onerous administration regimens and short half-lives. However the HDAC inhibitor class of drug continues to hold great promise. By focusing on a subset of HDAC inhibition that is more specific, and thus allows for the induction of HbF without alteration of cell cycle, we aim to identify an opportunity for combination therapy.
There are currently two open clinical trials treating sickle cell disease patients with more potent HDAC inhibitors. At our institution, a trial is open to treat SCD patients with vorinostat, and the first five patients have experienced no significant adverse effects (personal communication, Okam). Additionally, a similar study is open to treat adults with SCD with panobinostat, also known as LBH589 (NCT01245179). In both these trials, intolerance or refractoriness to hydroxyurea is an inclusion criteria, and patients cannot simultaneously take HU. The exclusion of patients taking hydroxyurea is very common in SCD clinical trials, but it is problematic. It would be optimal to include patients who are already taking hydroxyurea for several reasons. First, many patients derive a hematologic benefit from hydroxyurea, such as a modest increase in HbF, but continue to suffer from pain crises, acute chest syndrome, or other clinical manifestations of SCD5,30,31. An additional HbF induction agent could provide further clinical benefit. Second, a trial of a new drug may have a decreased likelihood of revealing a benefit if enrollment in the trial is limited to those patients who “fail” or refuse hydroxyurea. Patients with SCD are phenotypically heterogeneous with respect to their HbF levels, both at baseline, and in response to drugs that induce HbF. Although some patients who fail hydroxyurea may instead respond to a selective HDAC inhibitor, it is possible that a subgroup of patients who are poor hydroxyurea responders would also be less likely to respond to other HbF induction agents.
The predicted off-target clinical effects of HDAC inhibitors have been well documented. The dose-limiting toxicities are mainly non-hematologic, including anorexia, dehydration, diarrhea, and fatigue. Hematologic toxicity is most commonly thrombocytopenia, usually mild to moderate in severity. In contrast, the most common dose-limiting toxicity of hydroxyurea is neutropenia, and other toxicities include leg ulcers, mild changes in the hair and nails, mild gastrointestinal symptoms. Since these are largely non-overlapping, each agent could reach its maximum tolerated dose and yield optimal combined effect.
In conclusion, knockdown of HDAC1 or HDAC2 in human hematopoietic stem and progenitor cells is sufficient to induce gamma globin expression and has no effect on proliferation or p21 induction. In addition, hydroxyurea and an HDAC inhibitor can be combined in vitro at low doses to achieve a greater degree of γ-globin induction than either compound alone. The results of this report provide evidence to support a clinical trial of HDAC inhibitors in combination with hydroxyurea in patients with SCD.
Supplementary Material
Acknowledgments
The authors thank Damien Wilpitz for general laboratory support. This work was supported by the National Institutes of Health U01HL117720 and the Doris Duke Charitable Foundation. E.E. was supported by a Harvard Blood Scholars K12 award, sponsored by the National Heart, Lung, and Blood Institute of the NIH.
Footnotes
Authorship Contributions
E.E. and B.L.E. designed the experiments; E.E., M.M., K.L., and A.F. performed the experiments; E.E., M.M., K.L., A.F., and B.L.E. analyzed data; and E.E. and B.L.E wrote the paper.
Disclosures of Conflicts of Interest
The authors declare no competing financial interests.
References
- 1.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757):2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- 2.Heeney MM, Ware RE. Hydroxyurea for children with sickle cell disease. Hematol Oncol Clin North Am. 2010;24(1):199–214. doi: 10.1016/j.hoc.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337(11):762–769. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- 4.Platt OS, Orkin SH, Dover G, Beardsley GP, Miller B, Nathan DG. Hydroxyurea enhances fetal hemoglobin production in sickle cell anemia. J Clin Invest. 1984;74(2):652–656. doi: 10.1172/JCI111464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332(20):1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg MH, Barton F, Castro O, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. 2003;289(13):1645–1651. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 7.Steinberg MH, McCarthy WF, Castro O, et al. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: A 17.5 year follow-up. Am J Hematol. 2010;85(6):403–408. doi: 10.1002/ajh.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voskaridou E, Christoulas D, Bilalis A, et al. The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: results of a 17-year, single-center trial (LaSHS) Blood. 2010;115(12):2354–2363. doi: 10.1182/blood-2009-05-221333. [DOI] [PubMed] [Google Scholar]
- 9.Kinney TR, Helms RW, O'Branski EE, et al. Safety of hydroxyurea in children with sickle cell anemia: results of the HUG-KIDS study, a phase I/II trial. Pediatric Hydroxyurea Group. Blood. 1999;94(5):1550–1554. [PubMed] [Google Scholar]
- 10.Ferster A, Tahriri P, Vermylen C, et al. Five years of experience with hydroxyurea in children and young adults with sickle cell disease. Blood. 2001;97(11):3628–3632. doi: 10.1182/blood.v97.11.3628. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman SA, Schultz WH, Davis JS, et al. Sustained long-term hematologic efficacy of hydroxyurea at maximum tolerated dose in children with sickle cell disease. Blood. 2004;103(6):2039–2045. doi: 10.1182/blood-2003-07-2475. [DOI] [PubMed] [Google Scholar]
- 12.Ware RE, Eggleston B, Redding-Lallinger R, et al. Predictors of fetal hemoglobin response in children with sickle cell anemia receiving hydroxyurea therapy. Blood. 2002;99(1):10–14. doi: 10.1182/blood.v99.1.10. [DOI] [PubMed] [Google Scholar]
- 13.DeSimone J, Heller P, Hall L, Zwiers D. 5-Azacytidine stimulates fetal hemoglobin synthesis in anemic baboons. Proc Natl Acad Sci U S A. 1982;79(14):4428–4431. doi: 10.1073/pnas.79.14.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ley TJ, DeSimone J, Anagnou NP, et al. 5-azacytidine selectively increases gamma-globin synthesis in a patient with beta+ thalassemia. N Engl J Med. 1982;307(24):1469–1475. doi: 10.1056/NEJM198212093072401. [DOI] [PubMed] [Google Scholar]
- 15.DeSimone J, Koshy M, Dorn L, et al. Maintenance of elevated fetal hemoglobin levels by decitabine during dose interval treatment of sickle cell anemia. Blood. 2002;99(11):3905–3908. doi: 10.1182/blood.v99.11.3905. [DOI] [PubMed] [Google Scholar]
- 16.Perrine SP, Ginder GD, Faller DV, et al. A short-term trial of butyrate to stimulate fetal-globin-gene expression in the beta-globin disorders. N Engl J Med. 1993;328(2):81–86. doi: 10.1056/NEJM199301143280202. [DOI] [PubMed] [Google Scholar]
- 17.Liakopoulou E, Blau CA, Li Q, et al. Stimulation of fetal hemoglobin production by short chain fatty acids. Blood. 1995;86(8):3227–3235. [PubMed] [Google Scholar]
- 18.Atweh GF, Sutton M, Nassif I, et al. Sustained induction of fetal hemoglobin by pulse butyrate therapy in sickle cell disease. Blood. 1999;93(6):1790–1797. [PMC free article] [PubMed] [Google Scholar]
- 19.Cao H, Stamatoyannopoulos G, Jung M. Induction of human gamma globin gene expression by histone deacetylase inhibitors. Blood. 2004;103(2):701–709. doi: 10.1182/blood-2003-02-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao H, Stamatoyannopoulos G. Histone deacetylase inhibitor FK228 is a potent inducer of human fetal hemoglobin. Am J Hematol. 2006;81(12):981–983. doi: 10.1002/ajh.20676. [DOI] [PubMed] [Google Scholar]
- 21.Dover GJ, Brusilow S, Charache S. Induction of fetal hemoglobin production in subjects with sickle cell anemia by oral sodium phenylbutyrate. Blood. 1994;84(1):339–343. [PubMed] [Google Scholar]
- 22.Reid ME, El Beshlawy A, Inati A, et al. A double-blind, placebo-controlled phase II study of the efficacy and safety of 2,2-dimethylbutyrate (HQK-1001), an oral fetal globin inducer, in sickle cell disease. Am J Hematol. 2014;89(7):709–713. doi: 10.1002/ajh.23725. [DOI] [PubMed] [Google Scholar]
- 23.Bradner JE, Mak R, Tanguturi SK, et al. Chemical genetic strategy identifies histone deacetylase 1 (HDAC1) and HDAC2 as therapeutic targets in sickle cell disease. Proc Natl Acad Sci U S A. 2010;107(28):12617–12622. doi: 10.1073/pnas.1006774107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sowa Y, Orita T, Hiranabe-Minamikawa S, et al. Histone deacetylase inhibitor activates the p21/WAF1/Cip1 gene promoter through the Sp1 sites. Ann N Y Acad Sci. 1999;886:195–199. doi: 10.1111/j.1749-6632.1999.tb09415.x. [DOI] [PubMed] [Google Scholar]
- 25.Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci U S A. 2000;97(18):10014–10019. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.New M, Olzscha H, La Thangue NB. HDAC inhibitor-based therapies: can we interpret the code? Mol Oncol. 2012;6(6):637–656. doi: 10.1016/j.molonc.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moffat J, Grueneberg DA, Yang X, et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124(6):1283–1298. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 28.Dampier C, LeBeau P, Rhee S, et al. Health-related quality of life in adults with sickle cell disease (SCD): a report from the comprehensive sickle cell centers clinical trial consortium. Am J Hematol. 2011;86(2):203–205. doi: 10.1002/ajh.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panepinto JA, O'Mahar KM, DeBaun MR, Loberiza FR, Scott JP. Health-related quality of life in children with sickle cell disease: child and parent perception. Br J Haematol. 2005;130(3):437–444. doi: 10.1111/j.1365-2141.2005.05622.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG) Lancet. 2011;377(9778):1663–1672. doi: 10.1016/S0140-6736(11)60355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ware RE, Helms RW, Investigators SW. Stroke With Transfusions Changing to Hydroxyurea (SWiTCH) Blood. 2012;119(17):3925–3932. doi: 10.1182/blood-2011-11-392340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.