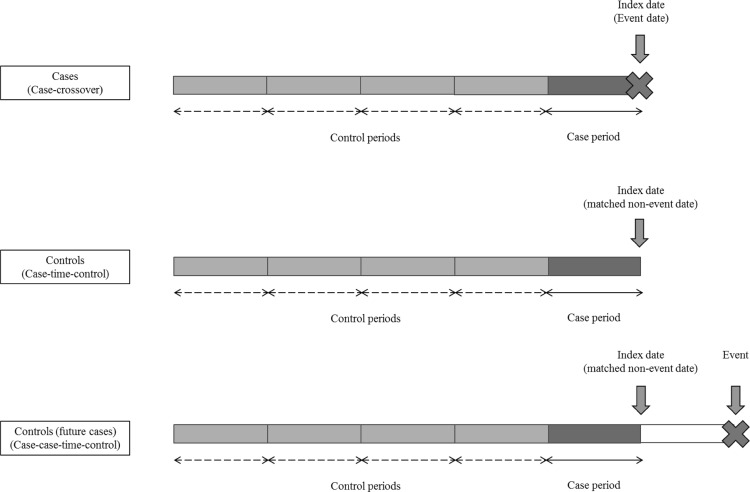
Fig. 3.
Case-crossover, case–time–control, and case–case–time–control study designs. In a case-crossover study, each case acts as a self-control from previous experience. Case period is defined as the time just before the occurrence of outcome event, while the control period is defined as the time preceding the case period. The drug exposure status during the case period is compared to that during the control period. In a case–time–control study, non-cases are sampled as controls to estimate the effect of exposure time-trend among the cases. Case–case–time–control study is an extension of a case–time–control study, where controls are sampled from future cases instead of non-cases