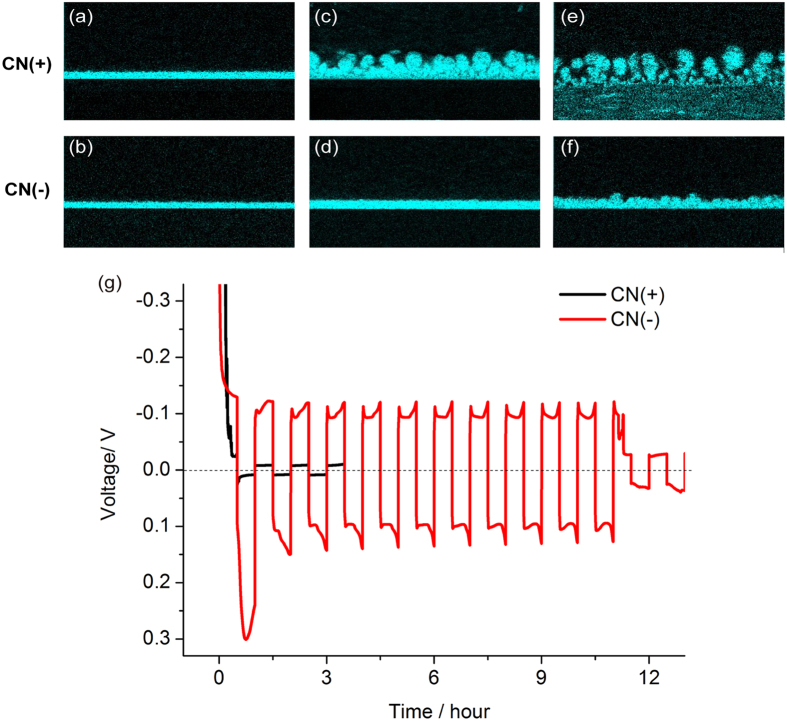
Figure 6. EDS mapping analysis of Cu element.
Cu is electrodeposited in CN(+) (a,c,e) and CN(−) (b,d,f) membranes at constant current densities in 100 mM CuSO4 for 2000s (a,b) −15 mA, (c,d) −20 mA, and (e,f) −25 mA. (g) Galvanostatic cycling profiles of CN(+) and CN(−) using a symmetric copper cell: Cu is electrodeposited and electrodissolved under extreme OLC (25 mA) for 1800 s in 100 mM CuSO4.