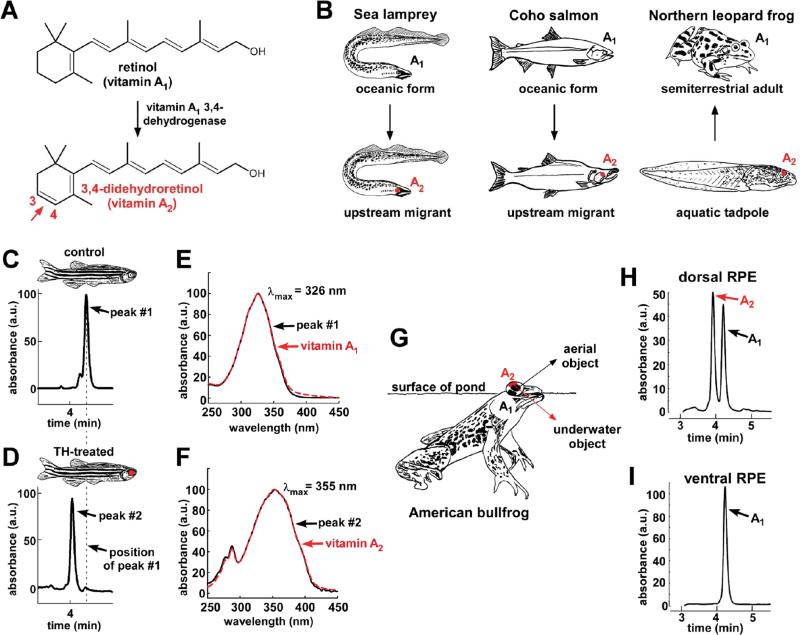
Figure 1. Modeling the rhodopsin-porphyropsin switch.
(A) The conversion of retinol (vitamin A1) to 3,4-didehydroretinol (vitamin A2) underlies the rhodopsin-to-porphyropsin switch and is driven by a previously unidentified dehydrogenase that catalyzes the addition of a double bond to the β-ionone ring.
(B) This vitamin A1 to A2 switch is widely used by a variety of vertebrates, and can be deployed at key stages of the life cycle: e.g., during upstream migration in sea lamprey (Petromyzon marinus) and Coho salmon (Oncorhynchus kisutch) and upon metamorphosis in amphibians such as the Northern leopard frog (Lithobates pipiens) [13-15].
(C, D) To model this switch, zebrafish were treated with thyroid hormone (300 μg/L L-thyroxine [T4]) for three weeks. Retinoids were then isolated from pooled RPE and retina of three individuals, reduced using sodium borohydride and analyzed by HPLC. Retinoids from TH-treated fish have a shorter retention time than those from vehicle-treated fish.
(E) The absorbance spectrum of the predominant retinoid from control fish closely matches the absorbance spectrum of a vitamin A1 standard, with a λmax of 326 nm.
(F) The predominant retinoid from TH-treated fish has an absorbance spectrum that matches that of the vitamin A2 standard, with a λmax of 355 nm.
(G) The American bullfrog (Lithobates catesbeianus) often sits at the water's surface and possesses exclusively A1-based visual pigments in the ventral retina and a mixture of A1- and A2-based visual pigments in the dorsal retina, possibly facilitating downward vision into the murky, red-shifted water [10, 19].
(H,I) The dorsal third of the RPE from two American bullfrogs was dissected, pooled, and analyzed by HPLC and found to contain a mixture of vitamin A1 and vitamin A2. Only vitamin A1 was identified in the ventral third of the RPE. All absorbance values are normalized and represented as arbitrary units (a.u.).