Figure 2.
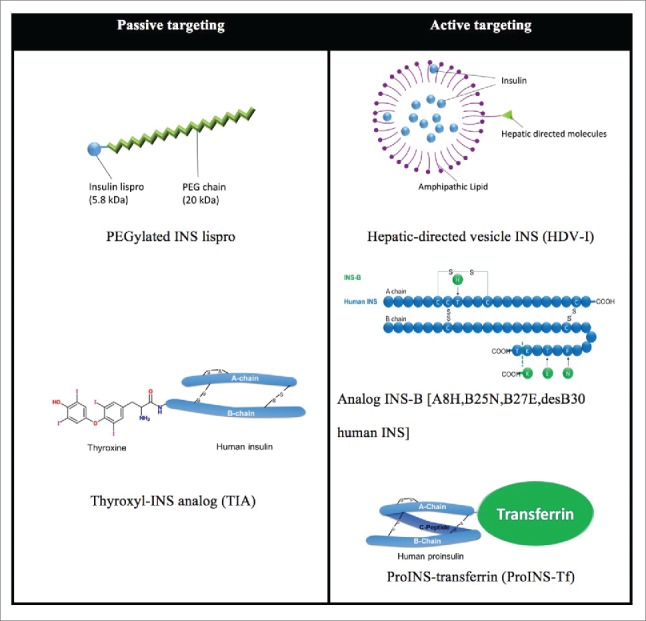
Two targeting approaches are exploited in the development of liver targeted INS therapy. Passive targeting by size increment of INS molecule (5.8 kDa) takes advantage of the different endothelial vascular sieves in peripheral tissues vs. the liver. Large molecules, which minimally diffuse through peripheral capillary walls, are freely filtered through fenestrated hepatic sinusoids. PEGylated INS lispro (Peglispro, LY2605541) is covalently coupled with a 20 kDa polyethylene glycol (PEG) and attains a hydrodynamic radius as large as a 71-98 kDa globular protein. Thyroxyl INS analog (TIA) is semi-synthesized by covalently linking thyronine to the ϵ-amino group of PheB1. In the active liver targeting area, hepatic-directed vesicle INS (HDV-I) encapsulates human INS in lipid vesicles (<150 nm in diameter) which have hepatocyte-targeting molecules (HTM) incorporated on the surface. HTMs include molecules targeting the asialoglycoprotein (galactose) receptor, hepatobiliary receptor or biotin receptor on hepatocytes. INS-B [A8H, B25N, B27E, desB30 human INS] is produced as an INS precursor in yeast with site directed mutagenesis, and the precursor is then enzymatically converted into 2-chain desB30 analogs. 30 ProINS-Tf is expressed in mammalian HEK293 cells by recombinant DNA techniques. MW increments of HDV-I and ProINS-Tf also presumably contribute to hepatoselectivity by passive targeting effect.
