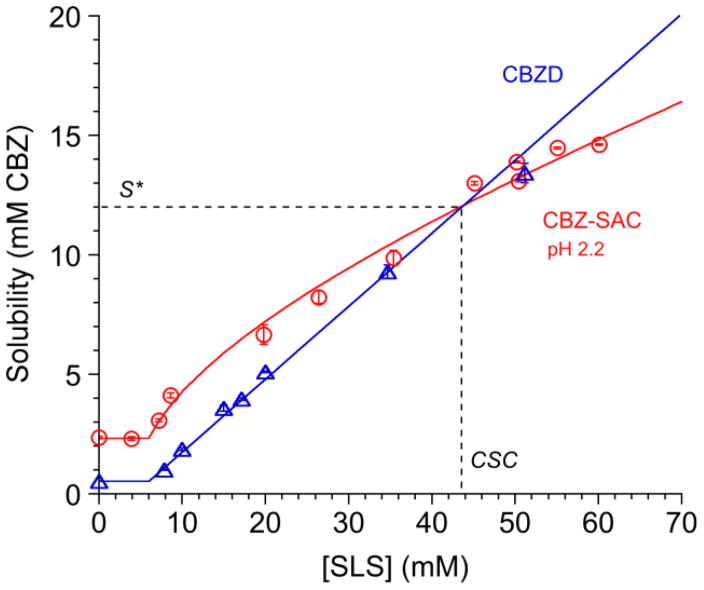
Fig. 13.
Cocrystal solubility values approach drug solubility values with increasing solubilizing agent concentration, as shown for the CBZ-SAC cocrystal and carbamazepine dihydrate (CBZD) in SLS solutions. Cocrystal solubility advantage over drug decreases with increasing drug solubilization, and can reach a value above which cocrystal is less soluble than drug. The intersection of solubility curves represents a transition point. This transition point is characterized by a solubility value (S*) where Scocrystal=Sdrug and a solubilizing agent concentration referred to as critical stabilization concentration (CSC). Cocrystal or drug solubilities above S* indicate that the cocrystal is above the transition point and drug is more soluble than cocrystal. Curves represent simulations according to the solubility equations for cocrystal and drug [69]. Adapted with permission from M. P. Lipert and N. Rodríguez-Hornedo from ref. [70]. Copyright 2015 American Chemical Society.