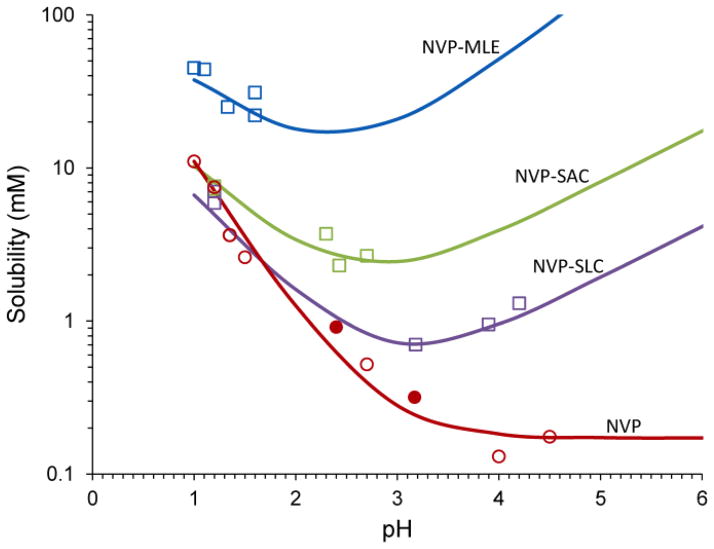
Fig. 14.
Solubility of the basic drug NVP and its cocrystals with acidic coformers: (1:1) cocrystal NVP-MLE, and (2:1) NVP-SAC and NVP-SLC as a function of pH. Symbols represent solubilities determined from solutions saturated with NVP and/or cocrystal at 25°C. pH values correspond to equilibrium pH. As pH in increased the cocrystal and drug solubility curves approach each other and intersect at pHmax. The pH value at the intersection of the drug and cocrystal (NVP-SAC and NVP-SLC) solubility curves corresponds to pHmax or transition point above which a less soluble cocrystal becomes more soluble than drug. Curves were calculated from cocrystal and drug solubility-pH dependence according to equations and and parameter values presented in the text and in Table 3. Symbols represent: NVP solubility (NVP hydrate-open circles, NVP anhydrous-filled circles) and cocrystal solubilities from eutectic points (squares). Reproduced from ref. [73] with permission from The Royal Society of Chemistry.