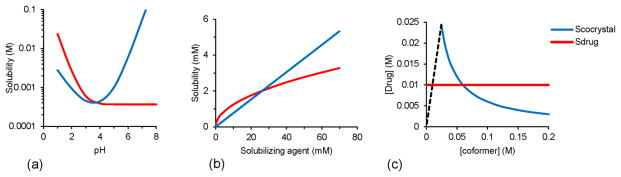
Fig. 2.
Cocrystal solubility can be fine-tuned by (a) pH, (b) drug solubilizing agents, and (c) coformer concentration, where dashed line represents stoichiometric concentrations of (1:1) cocrystal. Solution conditions change the cocrystal solubility relative to drug solubility and so the cocrystal thermodynamic stability. The cocrystal is thermodynamically stable when Scocrystal ≤ Sdrug. The cocrystal solubility advantage over drug (Scocrystal/Sdrug) when Scocrystal > Sdrug is however critical to achieve higher drug concentrations during cocrystal dissolution.