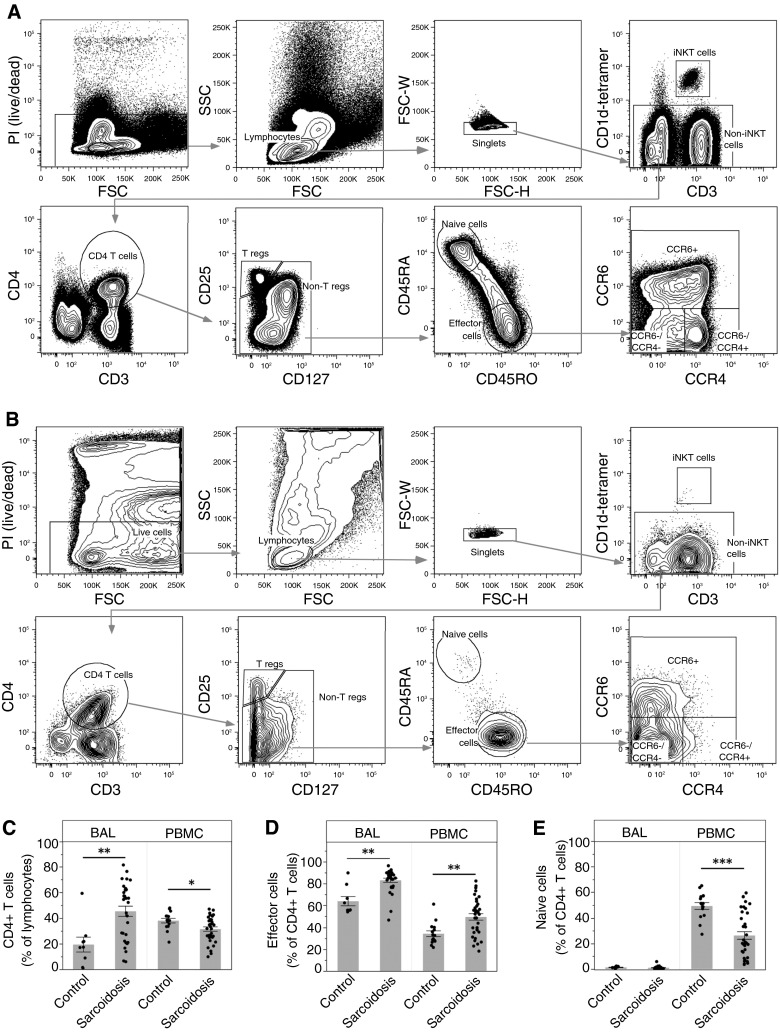
Figure 1.
Flow cytometry sorting strategy and comparative analysis of CD4+ T-cell subsets. Peripheral blood mononuclear cells (PBMCs) and bronchoalveolar lavage (BAL) cells were stained and analyzed by an LSRII Fortessa cytometer (BD Biosciences). (A and B) Representative sample is shown for blood (A) and BAL cells (B) from a patient with sarcoidosis. Lymphocytes were analyzed using the following series of subgates: live cells, lymphocytes, singlets, non–invariant natural killer (iNKT), CD4 T cells (CD4+CD3+), nonregulatory T cells (CD4+CD127+CD25− T cells), memory effector cells (CD45RA−/CD45RO+ CD4 T cells), and three subpopulations of memory effector cells using CCR4 and CCR6. We excluded iNKT cells (CD1d tetramer-PBS57), regulatory T cells (CD4+CD3+CD127−CD25+), and naive T cells (CD4+CD3+CD45RA+CD45RO−) from the CD4+ population. (C) CD4+ T cells as a percentage of lymphocytes are significantly increased in the lungs of patients with sarcoidosis (**P = 0.0019), and decreased in the blood (*P = 0.012). (D) Effector cells as a percentage of CD4+ T cells are significantly increased in both the lungs (**P = 0.0015) and the blood (**P = 0.0004) of patients with sarcoidosis. (E) There are essentially no naive cells in the lungs, and a significant decrease of naive cells as a percentage of CD4+ T cells in the blood of patients with sarcoidosis (***P < 0.0001). Data are expressed as mean (histogram bars) ± SEM. Overlaid dots represent individual patient values. BAL, n = 32 subjects with sarcoidosis and 9 control subjects; PBMC, n = 35 subjects with sarcoidosis and 18 control subjects. FSC = forward scatter; PI = propidium iodide; SSC = side scatter; T regs = T regulatory cells.