ABSTRACT
Therapies for liver cancer particularly those including radiation are still inadequate. Inhibiting the stress response machinery is an appealing anti-cancer and radiosensitizing therapeutic strategy. Heat-shock-protein-90 (HSP90) is a molecular chaperone that is a prominent effector of the stress response machinery and is overexpressed in liver cancer cells. HSP90 client proteins include critical components of pathways implicated in liver cancer cell survival and radioresistance. The effects of a novel non-geldanamycin HSP90 inhibitor, ganetespib, combined with radiation were examined on 3 liver cancer cell lines, Hep3b, HepG2 and HUH7, using in vitro assays for clonogenic survival, apoptosis, cell cycle distribution, γH2AX foci kinetics and client protein expression in pathways important for liver cancer survival and radioresistance. We then evaluated tumor growth delay and effects of the combined ganetespib-radiation treatment on tumor cell proliferation in a HepG2 hind-flank tumor graft model. Nanomolar levels of ganetespib alone exhibited liver cancer cell anti-cancer activity in vitro as shown by decreased clonogenic survival that was associated with increased apoptotic cell death, prominent G2-M arrest and marked changes in PI3K/AKT/mTOR and RAS/MAPK client protein activity. Ganetespib caused a supra-additive radiosensitization in all liver cancer cell lines at low nanomolar doses with enhancement ratios between 1.33–1.78. These results were confirmed in vivo, where the ganetespib-radiation combination therapy produced supra-additive tumor growth delay compared with either therapy by itself in HepG2 tumor grafts. Our data suggest that combined ganetespib-radiation therapy exhibits promising activity against liver cancer cells, which should be investigated in clinical studies.
KEYWORDS: Ganetespib, G2-M arrest, radiation therapy, Hsp90, liver cancer, radiosensitizer, stress response machinery
Introduction
Hepatocellular carcinoma (HCC) is a major global health concern. Although more than 80% of HCC cases are found in Africa and East Asia, there is a rising incidence of cases in the western world due to the contraction of hepatitis C in the 1960s and 1970s.1-4 The low 5-year survival rate of 10–15% can be attributed to the discovery of HCC in its advanced stages in the majority of cases and lack of effective therapies for advanced disease.5,6
Surgical tumor resection, with 5 y survival rates of 60%–70%, or transplantation, with 4 y survival rates of 70%–80%, are the preferred methods for HCC treatment, however only a minority of patients are candidates for surgery and recurrence is common.7 Non-surgical liver-directed therapies such as ablation, embolization, or radiation are used commonly, but many patients develop recurrent disease or present with an advanced stage of the disease which cannot be cured by these methods.6,8 Furthermore, HCC is relatively resistant to conventionally fractionated radiation9 and physical radiation dose escalation is greatly limited by normal liver tissue toxicity. Methods to dose escalate to liver cancer cells selectively would be advantageous as local control in the setting of compromised normal liver parenchyma is commonly a critical issue in many HCC patients.
Targeting the non-oncogene addiction or stress response machinery is gaining recognition as a potent cancer therapeutic strategy. In the high stress environment of cancers, tumor cells are subject to harsh conditions including hypoxia, inflammation, DNA damage and alterations in metabolic behavior.10 Cancer cell survival is maintained through the help of cellular stress response machinery such as heat shock proteins. Heat shock protein 90 (HSP90) is a molecular chaperone that assists in the post-translational folding and stabilization of many proteins, including various growth factor receptors and oncoproteins required for tumor maintenance. HSP90 has been reported to be overexpressed in HCC cell lines and HCC patient tumors when compared to the normal liver.8 Pre-clinical studies have provided a rationale for inhibition of HSP90 as an anti-cancer target for HCC, but combined effects of HSP90 inhibition with radiation are unknown. We have recently demonstrated targeting HSP90 with a second generation inhibitor potently radiosensitizes prostate cancer cells and delays prostate tumor growth in immunocompetent mice.11
The previous geldanamycin-analog generation of HSP90 inhibitors have shown modest results in several clinical trials, but limitations such as poor solubility, inconsistent pharmacokinetics, and hepatotoxicity, among other factors, have led to the development of newer and more potent class of HSP90 inhibitors.8,11 Ganetespib is a non-geldanamycin triazolone HSP90 inhibitor with single agent anti-cancer activity which has been shown to have superior pharmacodynamics compared to other HSP90 inhibitors.12 The drug has shown promise as an antitumor agent in phase II trials of patients with non-small cell lung cancer and metastatic breast cancer.13,14 For advanced HCC, a phase I pharmacokinetic study of ganetespib showed a very manageable safety profile, and established a recommended phase II dose,8 but combined effects of ganetespib with radiation are unknown. Herein we report potent in vitro and in vivo radiosensitizing effects of ganetespib on HCC cells and potential mechanisms of radiosensitization.
Results
Ganetespib (STA-9090) treatment induces radiosensitization of HCC cells in vitro
Hep3b, HepG2 and HUH7 cells were treated with increasing concentrations of ganetespib (0, 10, 50 and 100 nM) and all lines showed a dose dependent increase of HSP72 levels by western blotting (Fig. 1A), correlating with degree of HSP90 inhibition. HCC cell lines rely on several cellular pathways for proliferation and survival, the components of which are HSP90 client proteins, such as the RAS/MAPK and PI3K/AKT/mTOR pathways.15-17 Ganetespib caused down regulation of these signaling pathways in Hep3b, HepG2 and HUH7 cells in a dose dependent fashion (ganetespib 0, 10, 50 and 100 nM). In all 3 HCC cell lines treated with ≥50 nM ganetespib, pERK1/2 expression was either noticeably reduced or completely lost (Fig. 1A). Phospho-S6 was also seen to be down-regulated in the presence of ≥50 nM ganetespib in all 3 HCC cell lines. These results suggest reduced activity of both the RAS/MAPK and PI3K/AKT/mTOR pathways in HCC cell lines treated with ganetespib.
Figure 1.
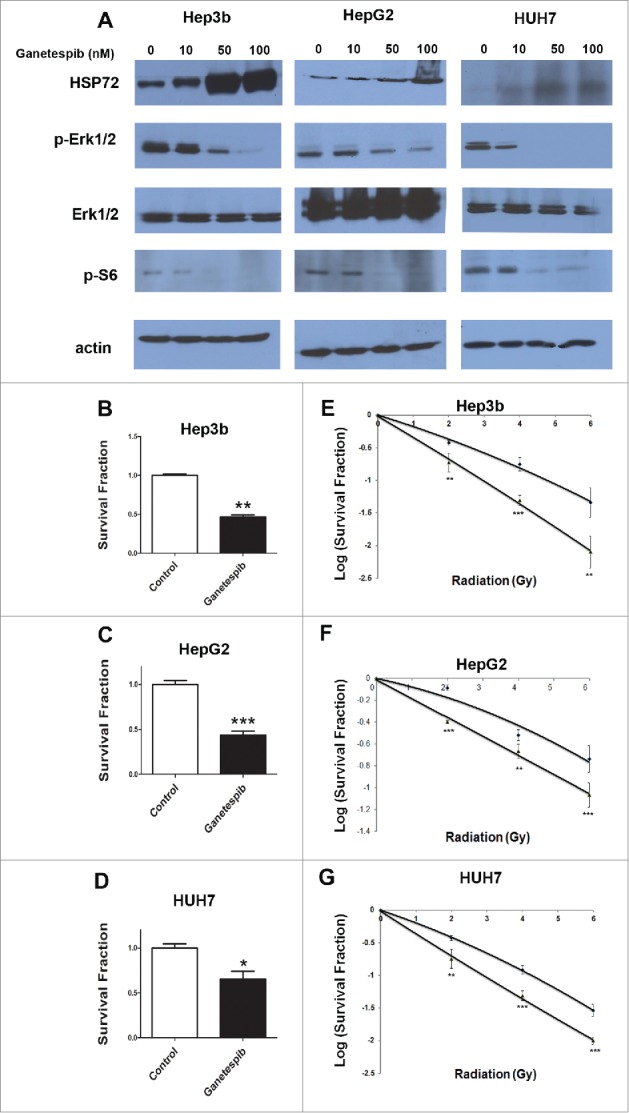
Ganetespib radiosensitizes hepatocellular carcinoma cell lines in vitro. (A) Cells were exposed to 24 hours of ganetespib at the specified concentration before protein extraction. Representative Western blotting showed a down-regulation of phospho-S6 and phospho-Erk1/2, but an upregulation of Hsp72. Total Erk1/2 levels remained constant throughout the treatment dosages. Clonogenic survival assay show the anticancer activity of ganetespib used as a single agent in (B) Hep3b, (C) HepG2, and (D) HUH7. Radiation clonogenic survival assays for (E) Hep3b, (F) HepG2, and (G) HUH7 demonstrated 5 nM ganetespib-induced radiosensitization in all 3 cell lines with enhancement ratios of 1.78, 1.33, and 1.47, respectively. All experiments were done in triplicate; error bars indicate SD and repeated at least twice. The * - p < 0.05, ** - p < 0.01 and *** - p < 0.001.
To assess the efficacy of ganetespib as a potential monotherapy and/or as a radiosensitizer for liver cancer we assayed the effect of ganetespib treatment on clonogenic potential of HCC cell lines. Clonogenic colony formation assays with Hep3b, HepG2 and HUH7 cell lines treated with ganetespib alone (5 nM) showed a reduction of 53.31%, 56.44% and 34.60% in survival fraction of Hep3b (Fig. 1B, p = 0.0032), HepG2 (Fig. 1C, p = 0.003) and HUH7 (Fig. 1D, p = 0.0172) cell lines, respectively.
Additionally, a greater decrease in survival fraction was found when the HCC cells treated with ganetespib were also subjected to ionizing radiation. The ganetespib enhancement ratios were 1.78 for Hep3b cells (Fig. 1E), 1.33 for HepG2 cells (Fig. 1F) and 1.47 for HUH7 cells (Fig. 1G). These data indicated that ganetespib is a potent inhibitor of clonogenic survival in HCC cells both as a single agent and also as a radiosensitizer.
Ganetespib causes G2-M arrest and apoptosis induction in HCC cell lines
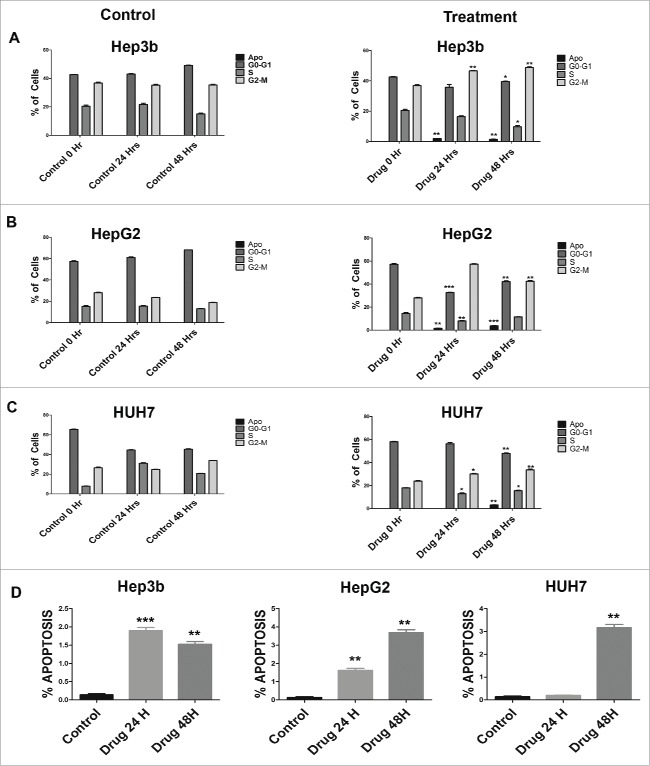
We synchronized HCC cells by serum starvation and treatment with aphidicolin and then subsequently treated them with ganetespib for 24 hours and analyzed for nuclear content by flow cytometry. Hep3b cells when treated with vehicle control (DMSO) did not show significant change in the cell cycle profile (Fig. 2A and Fig. S1) after 24 and 48 hours post treatment. However, ganetespib treated Hep3b cells show a marked increase in G2-M cells with 46.633% (SD 0.379) cells at 24 hours and 48.8% (SD 0.866) at 48 hours corresponding to a 11.373% and 13.4% increase over vehicle control at 24 and 48 hours, respectively (Fig. 2A). HepG2 cells showed the most relative arrest in G2-M in presence of ganetespib (Fig. 2B) with a 33.96% and 23.73% increase at 24 and 48 hours, respectively. A qualitatively similar pattern was observed for HUH7 cells (Fig. 2C). Taken altogether, ganetespib treatment caused HCC cells to undergo cell cycle arrest in G2-M, the most radiosensitive phase of the cell cycle.
Figure 2.
Cell cycle perturbations induced by ganetespib on HCC cells in vitro. Synchronized cells were exposed to vehicle control or 20 nM ganetespib for 0, 24, or 48 hours and then fixed with ethanol for cell cycle analysis with propidium iodide. Treatment with ganetespib caused a G2-M arrest in all cell lines as can be seen by the increase in percentage of cells in the G2-M phase at 24 and 48 hours when compared to vehicle controls for (A) Hep3b, (B) HepG2, and (C) HUH7 cell lines. Percent of cells in G1, S, G2 and G2–M phases is plotted for the control and ganetespib arms. (D) The sub-G1 population of cells as shown by flow cytometry of HCC cells treated with ganetespib (20 nM) or vehicle control for Hep3b, HepG2 and HUH7 cell lines. All experiments were done in triplicate and repeated at least twice. Error bars indicate SD. The * - p < 0.05, ** - p < 0.01 and *** - p < 0.001.
Treatment of HCC cell lines with ganetespib caused an increase of sub-G1 population of cells indicative of apoptosis. Hep3b, HepG2 and HUH7 cells showed a 1.365% (SD 0.035), 3.687% (SD 0.27) and 3.16% (SD 0.252) increase in cells sub-G1 cells by 48 hours, respectively (Fig. 2D). We confirmed the induction of apoptosis by ganetespib as shown by Western blotting showing increased cleaved caspase 3 (Fig. S2). These data suggest that ganetespib anti-cancer effects in HCC cell lines may be partially from induction of apoptosis.
Ganetespib delays repair of radiation induced double stranded breaks in HCC cells
To further explore mechanisms of ganetespib mediated radiosensitization of HCC cells, we examined dynamics of DNA double strand break repair (DSBR) in HCC treated cells. HCC cells were treated with DMSO control or with 20 nM of ganetespib, 24 hours post drug treatment the cells were subjected to 4 Gy of radiation and assessed for γH2AX foci formation indicative of DNA double strand breaks (DSBs). Hep3b (Figs. 3A and 3B), HepG2 (Fig. 3C and Fig. S3) and HUH7 (Fig. 3D and Figs. S3) control and ganetespib alone treated cells show very low basal levels of DSBs. Radiation alone and radiation-ganetespib treated cells as expected showed cells with high numbers of γH2AX foci at 30 minutes (p < 0.001, Fisher's exact test). At 24 hours following treatment, γH2AX foci were significantly reduced in all 3 HCC cell lines in the radiation alone arm (Fig. 3B-D), while cells treated with radiation-ganetespib still showed cells with high levels of γH2AX foci (p < 0.001 for Hep3b and HUH7 and p = 0.0002 for HepG2, Fisher's exact test).
Figure 3.
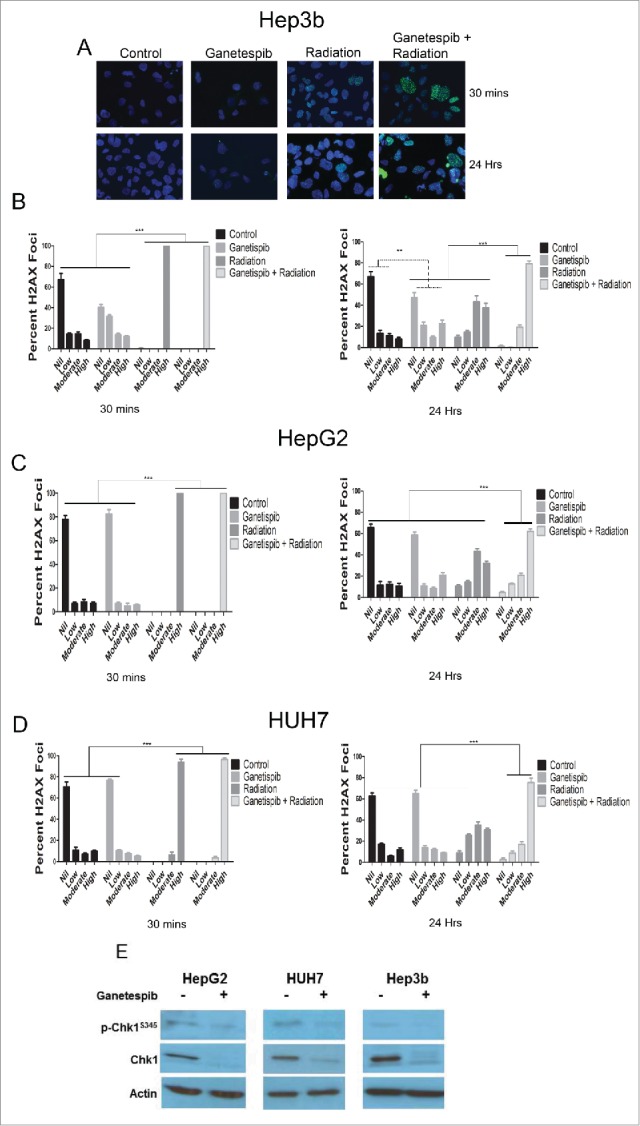
Ganetespib delays the repair of radiation-induced double strand breaks and downregulates the double strand break repair protein Chk1 in HCC cells. Immunofluorescence (IF) for γH2AX foci counterstained with DAPI and images captured using a fluorescent microscope. (A) Representative images are shown for Hep3B cell line at 30 minutes and 24 hours for each of the treatment arms; 20 nM ganetespib and/or 4 Gy of radiation when used. (B-D) Quantification of the percent of nuclei demonstrating no, low (<10 foci), moderate (10–25 foci) or high (>25) γ-H2AX foci per nuclei was quantified for all cell lines for each of the treatment arms and depicted graphically with standard error of the mean (SEM). For all cell lines, radiation alone and ganetespib-radiation resulted in a greater percentage of nuclei with a high number of γ-H2AX foci at 30 minutes when compared to any other treatment arm (p < 0.001, Fisher's exact test). At 24 hours, the ganetespib-radiation arm maintained a larger percentage of high γ-H2AX foci when compared to radiation alone (p < 0.001 for Hep3b and HUH7 and p = 0.0002 for HepG2, Fischer's exact test). (E) Cells were exposed to 24 hours of 50 nM ganetespib followed by an additional 24 hours prior to protein extraction and Western blotting for DNA damage response regulator p-Chk1-Ser345 and total Chk1 in all 3 HCC cell lines.
Probing for DNA damage response (DDR) machinery in HCC cell lines by western blotting showed that components and/or activity levels of the DDR, ATM, Chk1 and Wee1, were downregulated by ganetespib treatment. Phospho-Chk1-Ser345 and total Chk1 levels were downregulated in the presence of 50 nM ganetespib in all 3 HCC cell lines (Fig. 3E). In addition we observed total ATM and total Wee1 which are upstream and downstream DDR components, respectively, from Chk1 were similarly downregulated with 50 nM ganetespib treatment (Fig. S4). These results indicate that ganetespib radiosensitization of HCC cells may be explained by the prevention of repair of radiation-induced DSBs by inhibition of DDR client machinery.
Combined treatment with ganetespib and radiation delayed tumor growth in vivo
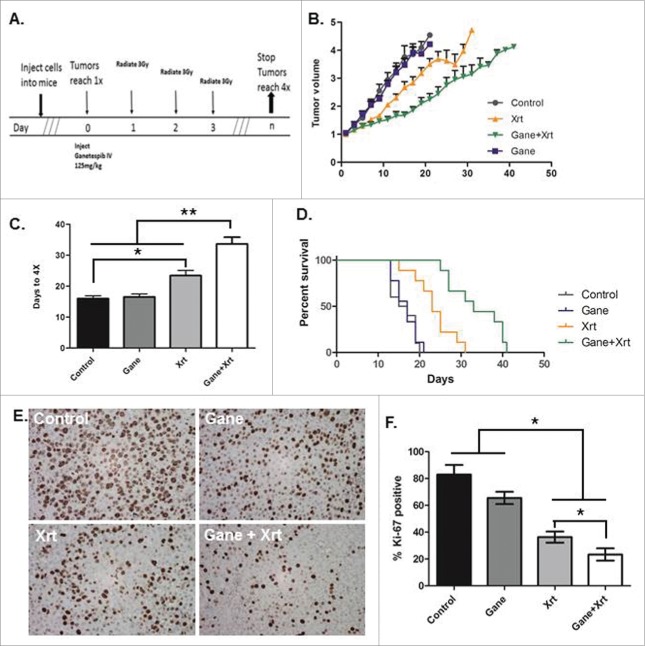
To assess the effects of ganetespib and fractionated radiation in vivo, HepG2 cells were implanted in the hind flanks of female nude mice with the treatment scheme shown in Fig. 4A. Combined radiation-ganetespib treatment delayed tumor volume growth (Fig. 4B) and quadrupling time compared with the single-treatment and control arms (Fig. 4C). The mean tumor quadrupling times were 16 ± 0.98 d for no treatment, 16.56 ± 0.92 d for ganetespib alone, 23.44 ± 1.63 d for radiation alone and 33.67 ± 2.173 d for the combined radiation-ganetespib treatment arm (Fig. 4C, p < 0.005 for radiation-ganetespib vs. any other arm by Mann-Whitney U test). HepG2 tumors treated with radiation-ganetespib required on average 17.67 d more to quadruple compared with untreated tumors. Interestingly, treatment with a single dose of ganetespib alone did not alter tumor progression, however, combined radiation-ganetespib exhibited greater than additive tumor growth delay of HepG2 tumors than seen with radiation alone (radiation-ganetespib 17.67 d > ganetespib 0.56 d + radiation 7.44 days). Similarly, using a Kaplan-Meier analysis with an event defined as time to tumor quadrupling, radiation-ganetespib resulted in significantly longer median time to quadrupling than the radiation alone treatment arm (Fig. 4D, p < 0.01, log-rank test). No observable differences in normal tissue toxicity, such as weight loss, diarrhea, dermatitis and ulceration, were noted between the combined radiation-ganetespib arm and either of the single-treatment arms (data not shown). Examining the xenograft tumors for proliferation by Ki67 IHC staining (Fig. 4E), the proliferative index was substantially decreased in radiation treated tumors (Fig. 4F, 46.37%, p = 0.0026 by Student's t-test) as compared to the DMSO control treated tumors (83%). Although a single dose of ganetespib treatment alone did not significantly alter the proliferative index of HepG2 cells in vivo (Fig. 4F,65.53%, p = 0.09 by Student's t-test), combined radiation-ganetespib treatment showed the least proliferative index of all treatments (Fig. 4F,32.34%, p < 0.05 by Students t-test for all arms). In summary, ganetespib demonstrated potent radiosensitization as measured by tumor growth delay of HCC cells in vivo.
Figure 4.
Ganetespib radiosensitizes HepG2 HCC cells and delays tumor growth in vivo in a supra-additive manner. A HepG2 hind-flank tumor growth delay model was used to assay (1) no treatment (Control), (2) ganetespib only (G or Gane), (3) fractionated radiation 3 Gy x 3 consecutive days (XRT), and (4) ganetespib and radiation (Gane-Xrt). (A) Treatment schema. The results were analyzed using (B) fold tumor volume change over time, (C) time to tumor quadrupling, and (D) Kaplan-Meier survival analysis where the event was considered time to tumor quadrupling. Ganetespib-radiation resulted in greater than additive tumor growth delay: ganetespib-radiation = 17.67 d > ganetespib alone = 0.56 d + radiation alone = 7.44 d. (E) Tumor samples were taken and analyzed by Ki67 IHC with representative images shown. (F) Right panel bar graph shows quantification of the percent Ki67 was significantly reduced in the combined ganetespib-radiation treatment arm compared to the other arms (p < 0.05 by t-test for pairwise comparisons of ganetespib-radiation versus all other arms). The * - p < 0.05, and ** - p < 0.005.
Discussion
Herein we have investigated the anti-tumor and radiosensitizing activity of ganetespib, a second generation HSP90 inhibitor, for liver cancer therapy. Ganetespib was able to reduce activity of multiple client signal transduction proteins believed to be important for liver cancer cell survival and proliferation, ERK1/2 and S6 proteins, in a dose dependent manner. We found that ganetespib treatment alone reduced clonogenic survival in all 3 HCC cell lines tested and further was able to radiosensitize these HCC cells in vitro. Treatment with ganetespib also resulted in increased apoptosis in HCC cells. Potential mechanisms for radiosensitization by ganetespib we observed were perturbations of the cell cycle resulting in increased numbers of cells in a G2-M arrest and decreased levels of the DSBR response protein CHK1, both of these findings were correlated with an increased number of unrepaired DSBs following irradiation. Finally, we showed that ganetespib was able to radiosensitize HCC cells to fractionated radiation in vivo using a tumor xenograft model.
HCC cells exhibits aberrant over expression and dysregulation of key cellular signaling molecules,18,19 exemplified by activation of the MAPK pathway20,21 and PI3K/AKT/mTOR pathway in 30–50% of cases.22 Sorafenib, a multi-kinase inhibitor is the only current effective systemic treatment for advanced HCC, but only has a modest durable response in most patients.23-25 HSP90 is known to be overexpressed in HCC cancers26,27 and thus, inhibition of HSP90 for treatment of HCC is a logical strategy as many oncoproteins important for cancer cell survival pathways are stabilized by HSP90. In addition, HSP90 inhibitors can target non-oncogene-dependent processes or stress response machinery that are also equally critical to the survival of HCC cells.
Ganetespib has already shown single agent pre-clinical activity against other cancers of the lung, prostate, colon and breast, as well as melanoma and leukemia cells in vitro and in some cases in vivo.28-30 A number of phase II and phase III clinical trials employing ganetespib are underway currently in a variety of non-HCC tumor histologies showing reasonable response rates.14 A recent phase I trial of ganetespib in advanced HCC patients showed a manageable toxicity profile even in patients with mild liver dysfunction.8 The radiosensitizing potential of ganetespib has also been recently demonstrated with lung cancer,31 stomach cancer32 and oropharyngeal squamous cell carcinoma.33
An important feature of HCC is local control of the tumor that frequently coexists with liver cirrhosis. This need has driven development of non-surgical options such as refinements in radiation therapy for HCC. However, HCC is relatively resistant to conventionally fractionated radiation9 and is limited by normal liver tissue toxicity. Thus, novel means to dose escalate to liver cancer cells selectively is an important area of research and positions ganetespib as an ideal candidate. First generation HSP90 inhibitors including geldanamycin analogs 17-AAG and 17-DMAG have been shown to radiosensitize a variety of tumor cells, but are plagued with prohibitive pharmacological and toxicity limitations.34-37 Second generation HSP90 inhibitors overcome these limitations and show similar potent radiosensitizing activity.11 Proposed mechanisms of HSP90 inhibitor-induced radiosensitization include inhibition of DSBR, as Chk1 and Rad51 are established HSP90 client proteins.38 Other non-mutually exclusive mechanisms include redistribution of cells into more radiosensitive phases of the cell cycle such as M-phase which appears to be a common effect of second generation HSP90 inhibitors. The effect of cell cycle distribution on radiosensitization is important in liver cancer cells as shown by concurrent versus sequential treatment of radiation with sorafenib.39
In summary we show that ganetespib inhibits HSP90 in a dose dependent manner leading to anti-cancer activity and potent radiosensitization of HCC cell lines by affecting key cellular signaling pathways, cell cycle arrest into more radiosensitive phases of the cell cycle, and inhibiting DNA repair mechanisms. These findings suggest ganetespib, when combined strategically with radiation, offers promise for liver cancer treatment and should be tested in clinical trials.
Materials and methods
Cell lines
Three human HCC cell lines, HepG2 (wild-type p53), HUH7 (Y220C-mutated p53; p21 deficient), and Hep3b (p53 and pRb deficient; hepatitis B virus positive) were used. Cells were grown and maintained in DMEM medium supplemented with 10% FBS and 1% penicillin-streptomycin. All cells will be incubated at 37°C in humidified 5% CO2. Cells were sub-cultured at 70–80% confluence and all experiments were carried out with the cells in an exponential growth phase. All cell lines were checked by short tandem repeat profiling and mycoplasma testing services of the Johns Hopkins Medicine Genetic Resources Core Facility.
Drug treatment
Ganetespib was obtained from Synta Pharmaceuticals Corp. Ganetespib (STA-9090) was dissolved in DMSO and stored at −20°C in 1 mM aliquots for the in vitro studies. For our in vivo experiments, the drug (dissolved in DMSO) was formulated in 20% Cremophor RH40 and 80% dextrose (5%) water and injected at 125 mg/kg by tail vein injection.
Radiation
For in vitro experiments, cells were irradiated with 0, 2, 4, or 6 Gy at room temperature using GammaCell irradiator with a 137Cs source at a dose rate of 50 cGy/min. For in vivo experiments, mice were treated using the Small Animal Radiation Research Platform (SARRP).40 The tumors were irradiated with a square beam of 1-cm × 1-cm with 3 consecutive daily fractions of 3 Gy.
Clonogenics assay
Cells in exponential growth phase were counted and plated in 10 cm dishes. Depending on the cell type, drug concentration, and radiation dose, 300–15,000 cells were plated. Ganetespib was added to the medium 24 hours after plating and radiation was delivered 24 hours after drug treatment. The drug was removed 24 hours post radiation by adding fresh growth medium. Colonies were stained and counted 10–14 d after irradiation by fixing with 0.1% Gentian Violet dissolved in a mixture of methanol and DI water in a 1:1 ratio. Colonies were counted under an inverted phase contrast microscope (Nikon Instuments Inc., Melville, NY) with a colony defined as comprising of at least 50 cells. Survival fraction was calculated as a function of plating efficiency. All arms were done in triplicate and repeated at least 3 times to ensure reproducibility.
Cell cycle analysis
For experiments with synchronized cells, 100,000–300,000 cells were seeded per well in 6-well plates allowed to attach in normal growth media for 24 hours, serum starved for 48 hours (0% serum), then grown in the presence of 10% serum and aphidicolin (2 μg/mL) for 24 hours before being released into normal growth medium (10% serum, without aphidicolin) containing ganetespib (20 nM). At various time points after adding ganetespib, cells were detached, washed with phosphate-buffered saline (PBS), and fixed with chilled 70% ethanol. Cells were pelleted and washed in PBS+1% BSA, then treated with 20 µg/mL RNAse-A with 10 µg/mL propidium iodide for 2 hours. DNA content was analyzed with FACSCalibur (BD Biosciences, Franklin Lakes, NJ) and FlowJo analysis software (Tree Star, Ashland, OR).
Immunoblot analysis
Cells were plated into 10 cm dishes and grown to sub confluence. Ganetespib (1–100 nM) was added to the medium 24 hours after plating. Cells were harvested, homogenized 24 hours later and 50 µg of total protein was loaded into each well of an 8–12% polyacrylamide gel and separated. Protein was transferred onto a polyvinylidene fluoride (BioRad) blotting membrane and blocked for an hour using 5% BSA or milk in TBST (tris-buffered saline supplemented with 0.1% Tween-20). Phospho-antibodies were incubated with 5% BSA in TBST, other antibodies were incubated with 5% milk in TBST. The membranes were probed with antibodies for phospho-S6 (Cell Signaling), ATM (Millipore), Wee1 (Cell Signaling), caspase 3 (Cell Signaling), phospho-Chk1-Ser345 (Santa Cruz), Chk1 (Santa Cruz), Hsp72 (Selleck chemicals), p42/44 (Cell Signaling), phospho-p42/p44 (Cell Signaling), and subsequently with horseradish peroxidase-labeled mouse anti-rabbit secondary antibodies (Sigma-Aldrich). Each antibody incubation step was followed by 3–4 washes with TBST. The secondary antibody was then coupled with GE ECL Plus kit (GE Life Sciences) and protein levels were detected using autoradiography films (Denville Scientific, Inc.). Experiments were done at least twice.
Immunofluorescence
Cells were plated on poly-L-lysine-coated (13.3 mg/ml) glass chamber slides/cover glass and incubated for 24 hours at 37°C in 5% CO2 and fixed for 15 minutes with freshly prepared 4% paraformaldehyde in phosphate-buffered saline (PBS). After washing with PBS, the cells were permeabilized for 15 minutes with PBST (PBS with 0.1% Triton X-100). The cells were then blocked with 2% FBS, 3% BSA(bovine serum albumin) in PBS for 30 minutes and incubated at room temperature for 1 hour with primary antibody(1:250) diluted in PBS. After washing with PBS, the cells were incubated with an Alexa Flour 488-conjugated secondary antibody (1:300, Molecular Probes, USA) for 1 hour at room temperature. Cells were washed in PBS and coverslips stained with DAPI prior to mounting. Fluorescent images were captured using a fluorescent confocal microscope. The cells were probed with primary antibodies for γH2AX (Cell Signaling).
Mouse tumor graft models and tumor growth delay experiments
Female athymic nude mice (Harlan, Indianapolis, IN) were maintained under pathogen-free conditions and given food/water ad libitum in accordance with Johns Hopkins Animal Care and Use Committee guidelines. Mice were injected subcutaneously in both flanks with 3×106 HepG2 cells in 100 µL of Hank's balanced salt solution and Matrigel (Invitrogen) mixed 1:1. Once tumors reached 100 mm3, 5–6 mice were randomly assigned to each of the 4 treatment arms: (1) no treatment; (2) ganetespib only; (3) radiation only and (4) ganetespib + radiation. Mice in ganetespib treated arms were given a single dose of 125 mg/kg, intravenously through the tail vein on the first day of treatment. The radiation arms were irradiated according to a fractionated scheme wherein, the mice were radiated for 3 consecutive days with a 3 Gy focal beam. Mice allocated to the combination arm were also subjected to the same fractionated radiation scheme as the radiation alone arm, with the first fraction of radiation being delivered 6 hours post drug treatment. The tumors were measured 3 times a week, until the tumors reached 4 times (4x) their pre-treatment volume. Tumor volume was calculated using the formula: length × width × height × π / 6. All experiments repeated twice.
Immunohistochemistry
Mice with established flank tumors from each treatment arm (1)-(4) above were sacrificed; for arms (3) and (4) in which radiation was a component of therapy, mice were sacrificed 1 hour after the first radiation delivery. Tumors were harvested, fixed in 10% formalin for 3 days, then transferred to PBS, fixed in paraffin, and sectioned by the Johns Hopkins Tissue Core Facility. Sections were stained for Ki67 (Abcam) as previously described using immunohistochemistry (IHC).41 The number of positively staining foci were counted and compared for at least 5 randomly chosen high power fields per tumor.
Statistics
Error bars included in graphical figures represent standard deviation (SD) unless otherwise specified. Two-tailed unpaired Student's t-test was used to compare cell cycle analysis, and Ki67 immunohistochemistry results between treatment arms. Fisher's exact test was used to compare γH2AX foci results between treatment arms. Clonogenic survival curves were fitted with a linear quadratic model using Microsoft Excel using a least squares fit, weighted to minimize the relative distances squared, and compared using the extra-sum of squares F test. Mean inactivation doses were determined using the method of Fertil42 and enhancement ratios calculated as the ratio of the mean inactivation dose for control vs. drug-treated arms as described by Morgan.43 A value significantly >1 indicates radiosensitization. Tumor growth delay assay results were compared by 2 distinct methods: (1) by using the log-rank test to compare median quadrupling times after creating a Kaplan-Meier plot using quadrupling in volume as the event of interest; and (2) by comparing quadrupling times between all tumors in 2 arms using the Mann-Whitney U test. A p-value of ≤0 .01 for 2-sided tests was considered significant.
Supplementary Material
Disclosure of potential conflicts of interest
David Proia is an employee of Synta Pharmaceuticals Corp makers of ganetespib. The remaining authors declare that they have no competing interests.
Contributions
SC, RM and AA participated in the design of the study, performed all the experiments, analyzed the data and drafted the manuscript. MB and SM helped with the Western blotting and clonogenic survival experiments. HW, JC and KT participated in the cell cycle and immunofluorescence studies. KM and YK assisted in the xenograft animal experiments. ZB, RA, DP, ML and JH helped draft the manuscript. PT conceived of the study, participated in its design coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Funding
H. Wailun was funded by Uniting Against Lung Cancer. K. Taparra was funded by the NIH (F31CA189588). J. Herman was funded by the Claudio X. Gonzalez Family Foundation, Flannery Family Foundation, Alexander Family Foundation, Keeling Family Foundation, DeSanti Family Foundation and McKnight Family. P. T. Tran was funded by the Keeling Family, the DoD (W81XWH-11-1-0272 and W81XWH-13-1-0182), a Kimmel Translational Science Award (SKF-13-021), an ACS Scholar award (122688-RSG-12-196-01-TBG) and the NIH (R01CA166348).
References
- 1.Weledji EP, Enow Orock G, Ngowe MN, Nsagha DS. How grim is hepatocellular carcinoma? Ann Med Surg 2012 2014; 3:71-6; PMID: 25568791; http://dx.doi.org/10861275 10.1016/j.amsu.2014.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Degos F, Christidis C, Ganne-Carrie N, Farmachidi JP, Degott C, Guettier C, Trinchet JC, Beaugrand M, Chevret S. Hepatitis C virus related cirrhosis: time to occurrence of hepatocellular carcinoma and death. Gut 2000; 47:131-6; PMID:10861275; http://dx.doi.org/ 10.1136/gut.47.1.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006; 144:70514; PMID: 16702586; http://dx.doi.org/23219567 10.7326/0003-4819-144-10-200605160-00004 [DOI] [PubMed] [Google Scholar]
- 4.Annemarie W, Scott G, Kathleen G. Centers for disease ontrol and prevention Surveillance for Acute Viral Hepatitis — United States, 2006. Wasington, D.C.: 2008. [Google Scholar]
- 5.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol Off J Am Soc Clin Oncol 2009; 27:1485-91; PMID: 19224838; http://dx.doi.org/23219567 10.1200/JCO.2008.20.7753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosemann S. Compass: hepatocellular carcinoma. OncoLog 2013; 58:1. [Google Scholar]
- 7.Klein J, Dawson LA. Hepatocellular carcinoma radiation therapy: review of evidence and future opportunities. Int J Radiat Oncol Biol Phys 2013; 87:22-32; PMID:23219567; http://dx.doi.org/ 10.1016/j.ijrobp.2012.08.043 [DOI] [PubMed] [Google Scholar]
- 8.Goyal L, Wadlow RC, Blaszkowsky LS, Wolpin BM, Abrams TA, McCleary NJ, Sheehan S, Sundaram E, Karol MD, Chen J, et al.. A phase I and pharmacokinetic study of ganetespib (STA-9090) in advanced hepatocellular carcinoma. Invest New Drugs 2015 Feb;33(1):128-37; PMID:25248753; http://dx.doi.org/ 10.1007/s10637-014-0164-8 [DOI] [PubMed] [Google Scholar]
- 9.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2002; 2:48-58; PMID:11902585; http://dx.doi.org/ 10.1038/nrc706 [DOI] [PubMed] [Google Scholar]
- 10.Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell 2009; 136:823-37; PMID:19269363; http://dx.doi.org/ 10.1016/j.cell.2009.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandhi N, Wild AT, Chettiar ST, Aziz K, Kato Y, Gajula RP, Williams RD, Cades JA, Annadanam A, Song D, et al.. Novel Hsp90 inhibitor NVP-AUY922 radiosensitizes prostate cancer cells. Cancer Biol Ther 2013; 14:347-56; PMID:23358469; http://dx.doi.org/ 10.4161/cbt.23626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ying W, Du Z, Sun L, Foley KP, Proia DA, Blackman RK, Zhou D, Inoue T, Tatsuta N, Sang J, et al.. Ganetespib, a unique triazolone-containing Hsp90 inhibitor, exhibits potent antitumor activity and a superior safety profile for cancer therapy. Mol Cancer Ther 2012; 11:475-84; PMID:22144665; http://dx.doi.org/ 10.1158/1535-7163.MCT-11-0755 [DOI] [PubMed] [Google Scholar]
- 13.Jhaveri K, Chandarlapaty S, Lake D, Gilewski T, Robson M, Goldfarb S, Drullinsky P, Sugarman S, Wasserheit-Leiblich C, Fasano J, et al.. A phase II open-label study of ganetespib, a novel heat shock protein 90 inhibitor for patients with metastatic breast cancer. Clin Breast Cancer 2014; 14:154-60; PMID:24512858; http://dx.doi.org/ 10.1016/j.clbc.2013.12.012 [DOI] [PubMed] [Google Scholar]
- 14.Socinski MA, Goldman J, El-Hariry I, Koczywas M, Vukovic V, Horn L, Paschold E, Salgia R, West H, Sequist LV, et al.. A multicenter phase II study of ganetespib monotherapy in patients with genotypically defined advanced non-small cell lung cancer. Clin Cancer Res Off J Am Assoc Cancer Res 2013; 19:3068-77; PMID: 23553849; http://dx.doi.org/22561517 10.1158/1078-0432.CCR-12-3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, Maad IB, Calderaro J, Bioulac-Sage P, Letexier M, Degos F, et al.. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet 2012; 44:694-8; PMID:22561517; http://dx.doi.org/ 10.1038/ng.2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y-W, Lin K-T, Chen S-C, Gu D-L, Chen C-F, Tu P-H, Jou Y-S. Overexpressed-eIF3I interacted and activated oncogenic Akt1 is a theranostic target in human hepatocellular carcinoma. Hepatol Baltim Md 2013; 58:239-50; PMID: 23460382; http://dx.doi.org/17295177 10.1002/hep.26352 [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Jin G, Jin H, Wang N, Luo Q, Zhang Y, Gao D, Jiang K, Gu D, Shen Q, et al.. Clusterin facilitates metastasis by EIF3I/Akt/MMP13 signaling in hepatocellular carcinoma. Oncotarget 2015 Feb 20;6(5):2903-16; PMID: 25609201; http://dx.doi.org/17295177 10.18632/oncotarget.3093; 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis 2007; 27:55-76; PMID:17295177; http://dx.doi.org/ 10.1055/s-2006-960171 [DOI] [PubMed] [Google Scholar]
- 19.Laurent-Puig P, Zucman-Rossi J. Genetics of hepatocellular tumors. Oncogene 2006; 25:3778-86; PMID:16799619; http://dx.doi.org/ 10.1038/sj.onc.1209547 [DOI] [PubMed] [Google Scholar]
- 20.Meloche S, Pouysségur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene 2007; 26:3227-39; PMID:17496918; http://dx.doi.org/ 10.1038/sj.onc.1210414 [DOI] [PubMed] [Google Scholar]
- 21.Crews CM, Alessandrini A, Erikson RL. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science 1992; 258:478-80; PMID:1411546; http://dx.doi.org/ 10.1126/science.1411546 [DOI] [PubMed] [Google Scholar]
- 22.Mínguez B, Tovar V, Chiang D, Villanueva A, Llovet JM. Pathogenesis of hepatocellular carcinoma and molecular therapies. Curr Opin Gastroenterol 2009; 25:186-94; PMID: 19387255; http://dx.doi.org/18650514 10.1097/MOG.0b013e32832962a1 [DOI] [PubMed] [Google Scholar]
- 23.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F, de Oliveira AC, Santoro A, Raoul J-L, Forner A, et al.. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378-90; PMID:18650514; http://dx.doi.org/ 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 24.Cheng A-L, Kang Y-K, Chen Z, Tsao C-J, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang T-S, et al.. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10:25-34; PMID:19095497; http://dx.doi.org/ 10.1016/S1470-2045(08)70285-7 [DOI] [PubMed] [Google Scholar]
- 25.Xie B, Wang DH, Spechler SJ. Sorafenib for treatment of hepatocellular carcinoma: a systematic review. Dig Dis Sci 2012; 57:1122-9; PMID:22451120; http://dx.doi.org/ 10.1007/s10620-012-2136-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Carbonero R, Carnero A, Paz-Ares L. Inhibition of HSP90 molecular chaperones: moving into the clinic. Lancet Oncol 2013; 14:e358-69; PMID:23896275; http://dx.doi.org/ 10.1016/S1470-2045(13)70169-4 [DOI] [PubMed] [Google Scholar]
- 27.Lamoureux F, Thomas C, Yin M-J, Fazli L, Zoubeidi A, Gleave ME. Suppression of heat shock protein 27 using OGX-427 induces endoplasmic reticulum stress and potentiates heat shock protein 90 inhibitors to delay castrate-resistant prostate cancer. Eur Urol 2014; 66:145-55; PMID:24411988; http://dx.doi.org/ 10.1016/j.eururo.2013.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Trepel JB, Neckers LM, Giaccone G. STA-9090, a small-molecule Hsp90 inhibitor for the potential treatment of cancer. Curr Opin Investig Drugs Lond Engl 2000 2010; 11:1466-76 [PubMed] [Google Scholar]
- 29.Proia DA, Blackman RK, Foley KP, He S, Kepros J, Korbut T, Sang J, Smith D, Ying W, Zhang C, et al.. The next generation Hsp90 inhibitor STA-9090, currently in phase 2 trials, displays potent in vitro and in vivo activity. Ann Oncol 2010; 21:ii35; PMID:22806877; http://dx.doi.org/21533169 10.1158/1078-0432.CCR-11-2967 [DOI] [Google Scholar]
- 30.Proia DA, Foley KP, Korbut T, Sang J, Smith D, Bates RC, Liu Y, Rosenberg AF, Zhou D, Koya K, et al.. Multifaceted intervention by the Hsp90 inhibitor ganetespib (STA-9090) in cancer cells with activated JAK/STAT signaling. PloS One 2011; 6:e18552; PMID:21533169; http://dx.doi.org/ 10.1371/journal.pone.0018552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomez-Casal R, Bhattacharya C, Epperly MW, Basse PH, Wang H, Wang X, Proia DA, Greenberger JS, Socinski MA, Levina V. The HSP90 inhibitor ganetespib radiosensitizes human lung adenocarcinoma cells. Cancers 2015; 7:876-907; PMID:26010604; http://dx.doi.org/ 10.3390/cancers7020814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu H, Lu J, Hua Y, Zhang P, Liang Z, Ruan L, Lian C, Shi H, Chen K, Tu Z. Targeting heat-shock protein 90 with ganetespib for molecularly targeted therapy of gastric cancer. Cell Death Dis 2015; 6:e1595; PMID:25590805; http://dx.doi.org/ 10.1038/cddis.2014.555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel K, Wen J, Magliocca K, Muller S, Liu Y, Chen ZG, Saba N, Diaz R. Heat shock protein 90 (HSP90) is overexpressed in p16-negative oropharyngeal squamous cell carcinoma, and its inhibition in vitro potentiates the effects of chemoradiation. Cancer Chemother Pharmacol 2014; 74:1015-22; PMID:25205430; http://dx.doi.org/ 10.1007/s00280-014-2584-8 [DOI] [PubMed] [Google Scholar]
- 34.Machida H, Matsumoto Y, Shirai M, Kubota N. Geldanamycin, an inhibitor of Hsp90, sensitizes human tumour cells to radiation. Int J Radiat Biol 2003; 79:973-80; PMID:14713575; http://dx.doi.org/ 10.1080/09553000310001626135 [DOI] [PubMed] [Google Scholar]
- 35.Bisht KS, Bradbury CM, Mattson D, Kaushal A, Sowers A, Markovina S, Ortiz KL, Sieck LK, Isaacs JS, Brechbiel MW, et al.. Geldanamycin and 17-allylamino-17-demethoxygeldanamycin potentiate the in vitro and in vivo radiation response of cervical tumor cells via the heat shock protein 90-mediated intracellular signaling and cytotoxicity. Cancer Res 2003; 63:8984-95; PMID:14695217 [PubMed] [Google Scholar]
- 36.Russell JS, Burgan W, Oswald KA, Camphausen K, Tofilon PJ. Enhanced cell killing induced by the combination of radiation and the heat shock protein 90 inhibitor 17-allylamino-17- demethoxygeldanamycin: a multitarget approach to radiosensitization. Clin Cancer Res Off J Am Assoc Cancer Res 2003; 9:3749-55; PMID: 1450616718645008 [PubMed] [Google Scholar]
- 37.Koll TT, Feis SS, Wright MH, Teniola MM, Richardson MM, Robles AI, Bradsher J, Capala J, Varticovski L. HSP90 inhibitor, DMAG, synergizes with radiation of lung cancer cells by interfering with base excision and ATM-mediated DNA repair. Mol Cancer Ther 2008; 7:1985-92; PMID:18645008; http://dx.doi.org/ 10.1158/1535-7163.MCT-07-2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He S, Smith DL, Sequeira M, Sang J, Bates RC, Proia DA. The HSP90 inhibitor ganetespib has chemosensitizer and radiosensitizer activity in colorectal cancer. Invest New Drugs 2014; 32:577-86; PMID:24682747; http://dx.doi.org/ 10.1007/s10637-014-0095-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wild AT, Gandhi N, Chettiar ST, Aziz K, Gajula RP, Williams RD, Kumar R, Taparra K, Zeng J, Cades JA, et al.. Concurrent versus sequential sorafenib therapy in combination with radiation for hepatocellular carcinoma. PloS One 2013; 8:e65726; PMID:23762417; http://dx.doi.org/ 10.1371/journal.pone.0065726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong J, Armour E, Kazanzides P, Iordachita I, Tryggestad E, Deng H, Matinfar M, Kennedy C, Liu Z, Chan T, et al.. High-Resolution, small animal radiation research platform with X-ray tomographic guidance capabilities. Int J Radiat Oncol 2008; 71:1591-9;PMID: 18640502; http://dx.doi.org/21974937 10.1016/j.ijrobp.2008.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tran PT, Bendapudi PK, Lin HJ, Choi P, Koh S, Chen J, Horng G, Hughes NP, Schwartz LH, Miller VA, et al.. Survival and death signals can predict tumor response to therapy after oncogene inactivation. Sci Transl Med 2011; 3:103ra99; PMID:21974937; http://dx.doi.org/ 10.1126/scitranslmed.3002018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fertil B, Dertinger H, Courdi A, Malaise EP. Mean inactivation dose: a useful concept for intercomparison of human cell survival curves. Radiat Res 1984; 99:73-84; PMID:6739728; http://dx.doi.org/ 10.2307/3576448 [DOI] [PubMed] [Google Scholar]
- 43.Morgan MA, Parsels LA, Kollar LE, Normolle DP, Maybaum J, Lawrence TS. The combination of epidermal growth factor receptor inhibitors with gemcitabine and radiation in pancreatic cancer. Clin Cancer Res Off J Am Assoc Cancer Res 2008; 14:5142-9; PMID: 18698032; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-4072 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.