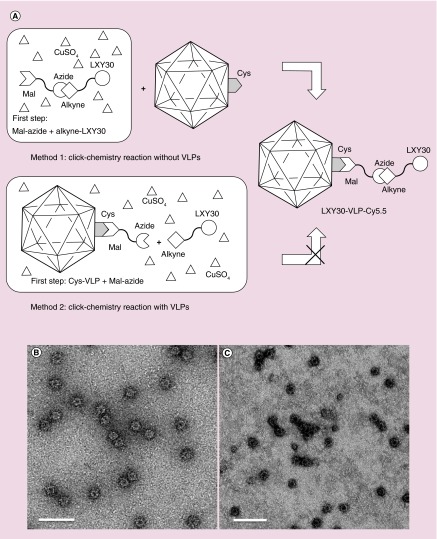
Figure 4. . The schematic of the two-step chemical conjugation process including a thiol-selective reaction and a copper catalyzed azide-alkyne cycloaddition or ‘click chemistry’ reaction to form LXY30-virus-like particles.
Two methods have been tested in this research. Method 1: the copper catalyzed azide-alkyne cycloaddition reaction between Mal-azide and alkyne-LXY30 to form Mal-linked LXY30 (Mal-LXY30) was done first. Then the Mal-LXY30 was added to react with Cys of N573C-VLPs (A). The LXY30 and Cy5.5 decorated N573C-VLP (LXY30-VLP-Cy5.5) remained intact after all these chemical conjugation processes (B). Method 2: the conjugation process starting by labeling Mal-linked azide (Mal-azide) at Cys sites of N573C-VLPs through thiol-selective reaction to build azide-linked VLPs (azide-VLPs), followed by adding LXY30-alkyne, ascorbic acid and CuSO4 to form LXY30-linked VLPs (LXY30-VLPs) (A). However, most LXY30-VLPs were damaged or disassembled from the conjugation processes, which may be caused by the reactive reactants including azide, ascorbic acid and CuSO4 (C).
Mal: Maleimide; VLP: Virus-like particle.