Abstract
BACKGROUND
Donor-specific antibodies (DSAs) to HLA antigens can cause acute antibody-mediated rejection (AMR) after kidney transplantation (Txp). Therapeutic plasma exchange (TPE) has been used for AMR treatment; however, DSA reduction rates are inconsistent. We investigated DSA reduction rates by HLA specificity and clinical outcome.
STUDY DESIGN AND METHODS
Sixty-four courses of TPE for 56 kidney Txp recipients with high DSA were investigated. Dates of TPE procedures and Txp, patients’ age, sex, race, creatinine (Cr), and mean fluorescent intensity (MFI) of DSA were retrieved. MFI reduction rate after one to three TPE and four to six TPE procedures were calculated by HLA DSA specificity in each patient, and the mean reduction rates were compared. The relationship of TPE treatment, MFI or Cr improvement rate, and graft age was also investigated.
RESULTS
Patients received a mean 6.0 TPE procedures. Most received intravenous immunoglobulin after TPE and immunosuppressives. Forty-two cases (65.6%) had DSA to HLA Class I and 54 cases (84.4%) to Class II, including 32 cases (50.0%) to both. Mean MFI reduction rates after one to three TPE and four to six TPE procedures were 25.7 and 37.1% in HLA Class I, 25.1 and 34.2% in Class II, and 14.3 and 19.9% in DR51-53. The mean Cr improvements at the end of TPE and 3 and 6 months after TPE were 3.41, −0.37, and −0.72%, respectively.
CONCLUSION
Six TPE procedures decreased DSA more than three TPE procedures, but reduction rate was lower by the second three TPE procedures than the first three TPE procedures. Although the mean Cr improvement was minimal, the treatment has good potential to stop further deterioration of kidney function. Better Cr improvement rate is correlated with the graft age.
Donor-specific antibodies (DSAs) to HLA antigens can cause acute antibody-mediated rejection (AMR) after kidney transplantation (Txp). In the past 20 years, high-dose pooled human intravenous immunoglobulin (IVIG) or therapeutic plasma exchange (TPE) followed by low-dose IVIG has been used to decrease DSA, immune complexes, or cytokines for pre-Txp desensitization to increase donor availability and to prevent hyperacute AMR. There have been many reports of successful HLA/ABO-incompatible kidney Txp with preoperative treatment by TPE followed by IVIG. AMR may be treated similarly, although controversy exists as to the number and timing of TPE and IVIG infusions. Recently, there have been significant advances with the technology to detect DSA. Multiplexed bead-based assays utilizing flow cytometric analysis are much more sensitive than previously widely used complement-dependent cytotoxicity method and allow for accurate determination of HLA DSA specificity and strength.
However, there are limited and inconsistent data regarding the efficacy of DSA reduction by TPE followed by IVIG, and the outcomes are uncertain for AMR treatment with TPE. The reduction rates of DSAs widely vary between patients and between DSA specificity. Zachary and colleagues1 reported that the elimination rates of all DSA by TPE and IVIG in pre-Txp desensitization are 75.6% for HLA Class I, 60.0% for Class II, and 20% for DR51-53. However, pre-Txp desensitization and post-Txp AMR treatment are the TPE applications for different clinical conditions and DSA responses to TPE may be very different. Preventing post-Txp hyperacute AMR by reducing DSA is the most important purpose for treatment on preoperative desensitization. On the other hand, kidney function recovery associated with DSA reduction is the goal for post-Txp AMR treatment. To better define optimal treatment strategy for AMR, we retrospectively investigated our experience with DSA reduction rate by HLA specificities and clinical outcome in post–renal Txp patients with AMR who underwent TPE followed by IVIG.
MATERIALS AND METHODS
Eighty-one courses of TPE were administered in 72 kidney Txp recipients for positive DSA between January 2009 and September 2012 at the University of Michigan Health System. Sixteen cases were excluded due to a lack of serial anti-HLA DSA determinations, an absence of HLA DSA (mean fluorescence intensities [MFIs] were <700 on all DSA reports), or an invasive procedure performed during the course of TPE treatments. A total of 64 treatment courses (cases) in 56 patients were investigated.
The patients underwent TPE treatment utilizing a uniform AMR protocol for kidney Txp consisting of one-plasma-volume exchange with 5% albumin replacement every other day for up to six procedures followed by 100 mg/kg sucrose-free isosmolar IVIG and 500 mg/kg after the last TPE. When a patient received an invasive procedure, such as renal biopsy, within 5 days before the first TPE, 5 to 10 mL/kg plasma was used as a replacement in addition to 5% albumin until 5 days after the procedure. A quantity of 500 mg/day methylprednisolone for 3 days was given if the patient did not require anti-thymocyte globulin for cellular rejection (no cellular rejection, borderline or Banff Classification 1A cellular rejection). If the patient had Banff Classification 2B or greater cellular rejection or steroid-resistant cellular rejection, the patient received 1.5 mg/kg anti-thymocyte globulin daily (maximum of 150 mg) for 7 to 10 days. All patients were on one or more kinds of immunosuppressives (steroid, mycophenolic acid mofetil, tacrolimus, cyclosporine, or rituximab) before and after the TPE treatments in the study period.
All patients were tested for antibodies to HLA-A, B, Cw, DR, DQ, and DP. These were tested by Luminex-based technology (Luminex, Luminex Corp., Austin, TX) using multiple HLA antigen-coated beads followed by single HLA antigen–coated beads. The strength of DSAs was reported as MFI, and MFI of less than 700 was considered as negative. For this analysis, we recorded negative MFI as 700.
Dates of TPE procedures were retrieved from the electronic medical record and MFI of all DSAs were from the tissue typing laboratory information system. Recorded data included patient age, date of Txp, and available creatinine (Cr) level on each day of TPE and at 3 months (within ±2 weeks) and at 6 months (within ±1 month) after the last TPE. The result of kidney biopsy was recorded. However, biopsy results were not considered for analysis since the histologic definition of AMR changed over the study period. Pretreatment MFI for each patient was the DSA result prior and closest to the first TPE procedure, within 5 days. Subsequent blood samples for DSA testing were drawn immediately before the third and sixth or the last TPE procedures to minimize the effect of IVIG on DSA results.
DSA results after the first, second, and third TPE procedures were grouped as one to three TPE procedures and that after the fourth, fifth, and the sixth TPE procedures was grouped as four to six TPE procedures. We designated time point (t-point) 0 as pretreatment, t-point 1 as after one to three TPE procedures, and t-point 2 as after four to six TPE procedures. The MFI reduction rate of each HLA specificity for each patient was calculated based on pretreatment MFI, grouped as above. DSA specificities DR51-53 were analyzed separately from other DR specificities due to their complexity of antigen, lower expression than the others, and a previous observation of higher persistence after TPE.1 Routine DSA testing to HLA Class II-DP started during the study period; therefore, the number of patients with DP DSA was smaller than that for other HLA specificities.
We investigated the relationship between the age of the transplanted kidney (graft age, in days) and MFI reduction rate of each DSA in HLA Class I and Class II after four to six TPE procedures (t-point 2). We grouped the cases into six categories, MFI reduction rates of not more than 0, 0 to 20, 20 to 40, 40 to 60, 60 to 80, and more than 80%, in each HLA class, and analyzed the corresponding median graft age in each group. We also grouped cases into two groups, MFI reduction rates of less than 60% and more than 60%, in each HLA class, and analyzed the relation to graft age.
We also investigated the relationship between the graft age and Cr improvement rate from available Cr immediately after the last TPE procedure (post-TPE), within 2 weeks of 3 months, and within 1 month of 6 months after the last TPE treatment in each case. Cr improvement rates were calculated based on Cr level within few days before the first TPE procedure started. In our facility, we treat patients who have subclinical AMR and high DSA or evidence of AMR on biopsy but normal Cr. For Cr improvement analysis, we excluded cases which had Cr of not more than 1.1 mg/dL at the time TPE procedure started and also during the observation period of 6 months from analysis because a Cr change within the reference range is not a clinical improvement. We grouped cases into six categories, Cr improvement rates of not more than 0, 0 to 20, 20 to 40, 40 to 60, 60 to 80, and more than 80% and analyzed the corresponding mean or median graft age in each group. We also grouped cases by graft age of not more than 1 year and more than 1 year and analyzed the Cr improvement rate for post-TPE, 3 and 6 months after the last TPE procedure.
We investigated the relationship of Cr improvement rate to duration of kidney dysfunction, calculated as the time in days between the date with last baseline Cr and the date of the first TPE procedure. The baseline Cr was set as normal Cr (≤1.1 mg/dL) for a patient whose Cr once had been not more than 1.1 mg/dL after the Txp or the last stable nadir Cr when the patient had never had normal Cr after the Txp. We grouped the cases into six categories by the same Cr improvement rate as above and analyzed the corresponding mean or median duration of graft dysfunction. We excluded cases with Cr levels of not more than 1.1 mg/dL in the observation period.
Frequencies were compared using chi-square tests. MFI data were not normally distributed and were compared using nonparametric Kruskal-Wallis and Wilcoxon signed-rank tests. Normally distributed Cr data were compared using t tests and Shapiro-Wilk test. Statistical analyses were performed using computer software (SYSTAT 13, Cranes Software International, Bangalore, India).
RESULTS
We identified 64 cases involving 56 patients (four patients were treated twice, two patients were treated 3 times). Twenty-nine patients were male (51.8%), and 27 patients were female (48.2%). Thirty-eight patients were Caucasian (67.9%), 13 patients were African American (23.2%), one patient was Asian (1.8%), and four patients were other races (7.1%). The mean age at the time of initiation of TPE was 39 (range, 11-70) years old. Patients received on average six procedures (range, two to 11).
A total of 214 DSAs were identified among the 64 cases. Ten cases (15.6%) had DSAs to only HLA Class I, 22 cases (34.4%) to only Class II, and 32 cases (50.0%) to both Class I and Class II. A total of 42 cases (65.6%) developed DSAs to Class I and a total of 54 cases (84.4%) developed DSAs to Class II. DSAs against HLA A, B, C, DR, DR51-53, DQ, and DP were found in 30 (46.9%), 23 (35.9%), 16 (25.0%), 31 (48.4%), 21 (32.8%), 35 (54.7%), and 10 (15.6%) cases, respectively (Table 1). Significantly more DSAs had Class II specificity (129, 60.3%) than Class I (85, 39.7%; p < 0.001). HLA DQ and DR51-53 antibodies had higher MFI on initial presentation than other specificities (Fig. 1, p < 0.001).
TABLE 1.
Distribution of DSA specificities among all patients
| HLA class | I | II | |||||
|
| |||||||
| Number of cases | 42 (65.6%) | 54 (84.4%) | |||||
| Number of DSAs | 85 (39.7%) | 129 (60.3%) | |||||
| Median MFI | 5,978.5 | 6,068.0 | |||||
|
| |||||||
| HLA specificity | A | B | C | DR* | DR51-53 | DQ | DP |
|
| |||||||
|
Number of
cases |
30 (46.9%) | 23 (35.9%) | 16 (25.0%) | 31 (48.4%) | 21 (32.8%) | 35 (54.7%) | 10 (15.6%) |
|
Number of
DSAs |
37 (17.3%) | 29 (13.6%) | 19 (8.9%) | 36 (16.8%) | 22 (10.3%) | 56 (26.2%) | 15 (7.0%) |
| Median MFI | 2,583.0 | 1,082.0 | 1,407.0 | 3,050.000 | 5,978.5 | 11,382.0 | 2,586.0 |
HLA DR specificities excluding DR51, DR52, and DR53.
Fig. 1.
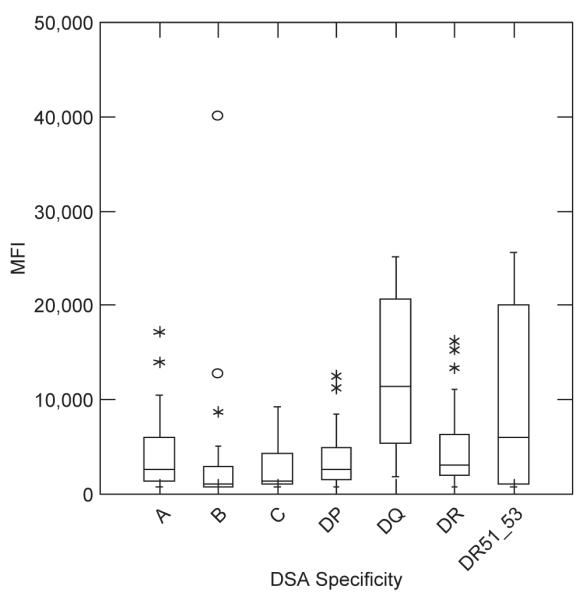
Distribution of pretreatment DSA MFI values by HLA specificity.
We compared the MFI values at each time point. Although MFI values were not normally distributed, the percent reduction values were normally distributed (p < 0.005). Table 2 shows the mean percent MFI reduction rate based on MFI values on t-point 0. Reductions were statistically significant for all specificities. However, we found that MFI reduction rate between t-point 1 and 2 based on MFI value at t-point 1 was much lower than that between t-point 0 and 1 for some specificities (Table 3). HLA Class I DSAs continued to decrease between t-points 1 and 2, but HLA Class II DSAs did not. Statistically significant differences in reduction between t-points 1 and 2 were seen for HLA A, B, and DP specificities, but not for HLA C, DR51-53, other DR, or DQ.
TABLE 2.
Mean MFI reduction percent based on MFI before the initiation of TPE (t-point 0)
| HLA I & II | |||||||
|
| |||||||
| t-point 1 | 25.3* | ||||||
| t-point 2 | 35.3* | ||||||
|
| |||||||
| HLA Class | I | II | |||||
|
| |||||||
| t-point 1 | 25.7* | 25.1* | |||||
| t-point 2 | 37.1* | 34.2* | |||||
|
| |||||||
| HLA Specificity | A | B | C | DR51-53 | Other DR | DQ | DP |
|
| |||||||
| t-point 1 | 40.8* | 6.9** | 25.2** | 14.3* | 36.8* | 22.0** | 28.2** |
| t-point 2 | 48.6* | 27.2* | 32.2* | 19.9* | 39.8* | 33.6* | 41.6* |
p < 0.005,
p < 0.05.
t-point 1: after one to three TPE treatments.
t-point 2: after four to six TPE treatments.
TABLE 3.
Mean MFI reduction rate on t-point 2 from t-point 1 value
| MFI reduction at | HLA I & II | ||||||
|
| |||||||
|
t-point 2 from
t-point 1 value |
2.7* | ||||||
|
| |||||||
| HLA Class | I | II | |||||
|
| |||||||
|
t-point 2 from
t-point 1 value |
8.8* | −2.3 | |||||
|
| |||||||
| HLA Specificity | A | B | C | DR51-53 | Other DR | DQ | DP |
|
| |||||||
|
t-point 2 from
t-point 1 value |
10.5** | 16.3** | 3.3 | −28.8 | 8.2 | −4.2 | 14.8** |
p < 0.005,
p < 0.05.
t-point 1: after one to three TPE treatments.
t-point 2: after four to six TPE treatments.
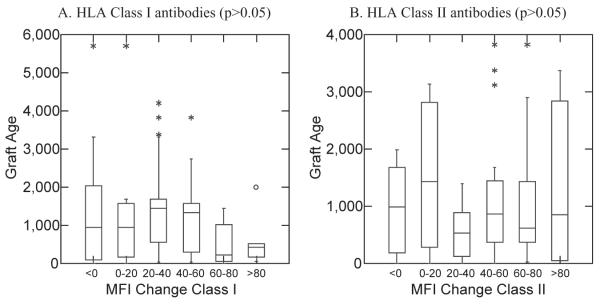
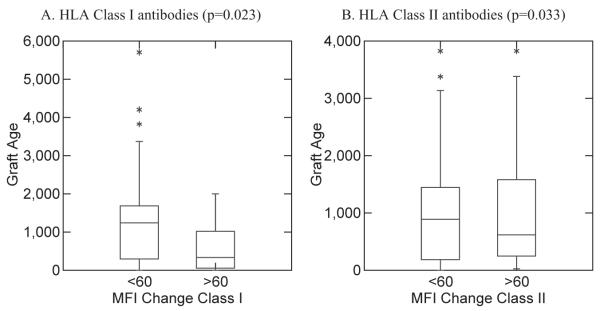
The mean graft age at the time of first TPE was 1286.2 days (range, 2-6547 days). The graft age grouped into six groups based on MFI reduction rate in HLA Class I and II at t-point 2 is shown in Fig. 2. There was no significant difference in MFI reduction rate and the graft age for both HLA Class I and II (p > 0.05). When MFI reduction rates at t-point 2 were grouped as less than 60% and more than 60%, there is a significant difference (p = 0.023 in HLA Class I, p = 0.033 in HLA Class II; Fig. 3).
Fig. 2.
Relationship between MFI reduction rate (six groups) and graft age at t-point 2 (after four to six TPE procedures).
Fig. 3.
Relationship between MFI reduction rate of less than 60% and more than 60% and graft age at t-point 2 (after four to six TPE procedures).
In the Cr improvement analysis, seven cases out of 64 cases were excluded because of normal Cr level at all observation time points. In 57 total cases, Cr data were available in all 57 cases for pre-TPE, in 57 cases for post-TPE, in 50 cases at 3 months after the last TPE, and in 43 cases at 6 months after the last TPE.
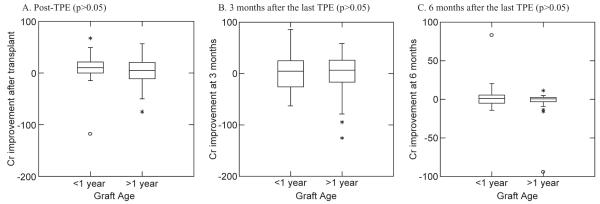
The overall mean Cr improvement rate was 3.4% (from −118.0% to 67.2%) at post-TPE, −0.37% (from −125.6% to 86.2%) at 3 months, and −0.72% (from −94.1% to 82.8%) at 6 months. The mean Cr improvement rates for the patients who received kidney Txp within 1 year before the initiation of AMR treatment at post-TPE and 3 and 6 months were 6.76, 6.09, and 6.02%, respectively, and for the patients who received kidney Txp more than 1 year after the initiation of AMR treatment were 1.69, −4.46, and −4.47%, respectively (Table 4). However, these differences were not significant (p > 0.05, Fig. 4).
TABLE 4.
Mean Cr improvement rate (%) after the TPE treatment based on graft age
| Cr improvement rate | After TPE |
3 months after TPE |
6 months after TPE |
|---|---|---|---|
| <1 year after Txp | 6.76 | 6.09 | 6.02 |
| >1 year after Txp | 1.69 | −4.46 | −4.47 |
| Overall | 3.41 | −0.37 | −0.72 |
Fig. 4.
Relationship between Cr improvement rate and graft age of less and more than 1 year at t-point 2 (after four to six TPE procedures).
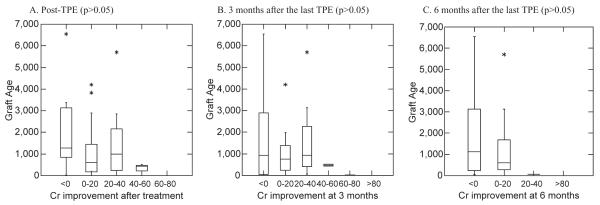
The mean graft age in six categories based on Cr improvement rate is shown in Table 5. The relationship between the median graft age and Cr improvement rate in these groups is shown in Fig. 5. Although there is no significant difference between groups (p > 0.05) at post-TPE and 3 and 6 months, a trend toward greater Cr improvement with younger grafts at any t-point is observed in Table 5.
TABLE 5.
Mean graft age (days) at the time of initiation of TPE treatment on Cr improvement rates
| Cr improvement rate (%) |
After TPE | 3 months after TPE |
6 months after TPE |
|---|---|---|---|
| ≤0 | 1462.5 | 1465.5 | 1813.2 |
| 0-20 | 1461.6 | 1340.2 | 1152.2 |
| 20-40 | 1047.3 | 1241.7 | 55.0 |
| 40-60 | 321.7 | 480.5 | |
| 60-80 | 7.0 | 10.0 | |
| >80 | 7.0 | 7.0 |
Fig. 5.
Relationship between Cr improvement rate and graft age (six groups).
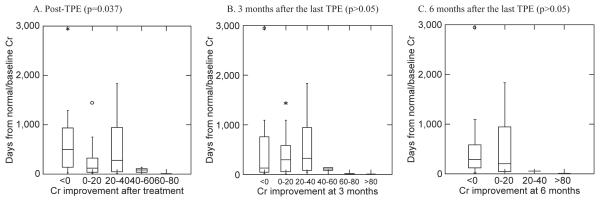
The mean days of graft dysfunction in six categories based on Cr improvement rate is shown in Table 6. The trend toward better Cr improvement in patients with shorter duration of graft dysfunction is also observed in this correlation study. The relationship between the median days of graft dysfunction and Cr improvement rate in these groups is shown in Fig. 6. There is a significant difference between groups at post-TPE (p = 0.037), but no significant difference at 3 and 6 months (p > 0.05).
TABLE 6.
Mean days of graft dysfunction at the time of initiation of TPE treatment on Cr improvement rates
| Cr improvement rate (%) |
After TPE | 3 months after TPE |
6 months after TPE |
|---|---|---|---|
| ≤0 | 548.0 | 511.5 | 499.7 |
| 0-20 | 319.0 | 411.3 | 491.0 |
| 20-40 | 547.2 | 563.3 | 55.0 |
| 40-60 | 72.3 | 106.5 | |
| 60-80 | 7.0 | 10.0 | |
| >80 | 7.0 | 7.0 |
Fig. 6.
Relationship between Cr improvement rate and the duration of graft dysfunction.
DISCUSSION
TPE is the choice for reduction of pathogenic antibodies before transplant, as well as for treatment of AMR. However, the reduction rates of DSAs by TPE followed by IVIG are not consistent, depending on the patient and on DSA specificity. This variability may be due to difference in the rate of DSA removal by TPE or in the rate of rebound. TPE removes all circulating immunoglobulins without regard to antigen specificity. Therefore, it is likely that the variability of overall reduction rates of each HLA specificity is due to difference in the rate of rebound.
We found more DSAs to HLA Class II specificities than to Class I (65.5% to Class I vs. 84.4% to Class II), which is somewhat higher than the result of the report by Zachary and colleagues2 (67.3% vs. 59.2%, respectively). In particular, we found that DSAs to DQ specificity was found in more than 50% of the patients (Table 1). Zachary and colleagues1 also reported elimination of all DSAs in 53.7% of patients who received treatment for pre-Txp desensitization. In our patient population, all DSAs were eliminated in only five of 64 cases (7.8%). The desensitization protocol employed by Zachary and colleagues and our protocol for AMR are similar. Notably, the highest DSA level on our five cases was only 2594 MFI. These differences between pretransplant desensitization and posttransplant treatment of AMR are explainable by the presence of active antigens on the transplanted kidney that may stimulate production of antibodies. This observation may suggest that post-Txp patients with AMR may require more aggressive immunosuppression to prevent rebound than patients for pre-Txp sensitization, such as higher doses of IVIG after TPE, medications for B-cell or plasma cell suppression, anti-thymocyte globulin for all AMR patients to suppress T-cell–dependent B-cell response, and a combination of those. This hypothesis is supported by a report by Ramos and coworkers,3 which showed that the combination therapy targeting multiple immune response pathways had greater effect in decreasing both mature and memory B cells in spleen.
We observed that DSA reduction was greater at four to six TPE procedures than one to three TPE procedures, although the rate was dependent on specificity. The DSA reduction rates for Class I and II and DR51-53 specificities at t-point 2 in our patients were 37.1, 34.2, and 19.9%, respectively. However, the reduction rate between t-points 1 and 2 was significantly lower than that between t-points 0 and 1 (25.3% between t-points 0 and 1 vs. 2.7% between t-points 1 and 2; Tables 2 and 3). This may be because the median DSA level was lower at the t-point 1 than at t-point 0. As Zachary and coworkers1 reported, DSA strength at initiation of treatment might have an impact on DSA elimination. We observed that HLA C, DR51-52, other DR, and DQ specificities did not show significant differences in MFI between t-points 1 and 2. Furthermore, we observed increases in HLA DR51-53, DQ and overall HLA II indicating that DSA rebound is higher during four to six TPE procedures in these specificities. This observation may suggest that continuation of TPE beyond an optimal number of procedures is detrimental for some DSA specificities.
The phenomenon of antibody rebound after TPE is well described. Dau4 reported a significant increase in B-cell percentage in peripheral blood lymphocyte populations with TPE, which leads to increasing antibody production. We hypothesize that at least four other mechanisms may account for rebound in the setting of TPE for AMR, as follows: 1) Naïve cells may be activated by TPE. When naïve cells are activated, they can be sensitized by existing antigens on transplanted kidney leading to production of de novo DSAs. Active antigens sensitize naïve cells more than inactive antigens, and our data on post-Txp patients with active antigen show lower overall DSA reduction rates than pre-Txp patients. 2) Memory B cells may be activated by TPE and produce more DSAs. Paglieroni and coworkers5 reported B cells are more activated 7 to 9 days after TPE. Although Sadeghi and colleagues6 showed that T and B lymphocyte counts were decreased immediately after TPE, the timing of these measurements may be too early to prove activation of cells. Additionally, since their TPE procedure used a filter method that is different from the centrifugation method we utilized, the rate of T- and B-cell activation may be different in our study. 3) Negative feedback on DSA-producing B cells is decreased by lowered DSA level leading to more DSA production. TPE removes existing DSAs and IVIG decreases DSA production. Plasma cells may not be affected by TPE and immunomodulatory medications as shown by Ramos and colleagues.3 Mayer and coworkers7 reported IVIG inhibits T-cell–dependent B-cell proliferation and differentiation, and Tawfik and colleagues8 suggested that IVIG inhibits T-cell proliferation leading to inhibition of T-cell–dependent B-cell proliferation. Therefore, when DSA levels are high, the immunoglobulin production from B cells is suppressed. However, when DSA levels are reduced by TPE and the down regulation effect of IVIG, negative feedback is decreased. The net result may be that B cells produce more DSAs after the effect of IVIG has diminished. 4) Antigens on damaged kidney tissue release bound antibodies (DSAs). Early in AMR, antigens on healthy kidney tissue absorb tissue-specific antibodies. At this point, circulating DSA level may be paradoxically low. However, when the graft tissue is damaged in AMR, the bound antibodies may be released into circulation leading to high DSA detection.
The trend toward better Cr improvement rate in patients with shorter graft age is seen in Tables 4 and 5. The patients with Cr improvement rate of more than 60% at any time point received their kidney transplants within 10 days before the treatment. In contrast, patients who received Txp 3 years or more before TPE treatment had overall Cr improvement rates of less than 20%. Further correlation study between the duration of graft dysfunction and Cr improvement showed a significant relationship between better Cr improvement and shorter duration of graft dysfunction (Fig. 6). The older grafts most likely had slow deterioration of graft function over a longer period in our study, possibly due to chronic rejection. This observation underscores the importance of early initiation of AMR treatment. It is difficult to detect deterioration of kidney function early long after the Txp because most patients’ post-Txp follow-up visits are usually less frequent over the time. Furthermore, the deterioration of graft function, and resultant Cr increase, may be too slow to trigger the treatment. Therefore, by the time that high DSA levels are detected, significant allograft injury may already have occurred, and the damage may be irreversible at the time of treatment.
The importance of early initiation of treatment is also highlighted by our correlation study between MFI improvement rate and graft age (Fig. 3) showing younger grafts had higher MFI improvement rate. It strongly suggests that early initiation of treatment is more effective to decrease MFI.
Assessing the correlation between Cr improvement rate and DSA reduction rate is problematic since 54 cases (84.4%) had multiple DSA specificities, and the role of individual DSA specificity in the causation of AMR in each patient is difficult to determine. For example, even though one high DSA specificity in a patient who has multiple types of high DSAs is significantly reduced by TPE treatment, Cr may not improve if that particular specificity is not playing a main role in AMR. Additionally, most of our patients had some degrees of cellular rejection and probably chronic rejection in older grafts, which may have some impact on the Cr result.
Overall, the mean Cr improvement rate was minimal at any point (Table 4) in our study. However, we observed that the Cr remained stable over 6 months of follow-up. Thus, TPE treatment may be able to halt further functional deterioration in older grafts for at least 6 months.
Recently, several studies have suggested that non-HLA antibodies may be responsible for some cases of AMR. In addition to improved sensitive HLA antibody detection, a test is available for the detection of C1q binding HLA antibodies,9-11 non-HLA DSAs including endothelial precursor cell antibodies, and polymorphic MHC Class I–related Chain A antibodies.12-15 Other non-HLA antibodies and autoantibodies, such as antibodies to anti-angiotensin II Type 1 receptor, anti-endothelin receptor,16 vimentin, tubulin, myosin, collagen,12 nucleolin,17 or laminin18 may also play important roles12 similar to the mechanisms to hemolysis caused by auto antibodies to RBCs. A recently available C1q binding HLA antibody detection may assist in the determination of which DSA specificities among multiple DSAs are causing AMR in future. However, the complexity of the test method and lower sensitivity to low titer of antibodies are limitations of the currently available tests.
Novel therapies for AMR, possibly in addition to TPE and IVIG are now becoming available. These include newer medications such as anti-CD20 for B cells (rituximab),19,20 proteasome inhibitors leading to plasma cell apoptosis (bortezomib21-24), anti-interleukin-2 receptor antibodies (daclizumab,25,26 basiliximab27,28), and anti-CD52 (alemtuzumab25,29). Anti-complement therapy (eculizumab,19,30,31 recombinant human C1 esterase inhibitor19,30,32-34) may also be possibilities in the future to interfere with the complement cascade initiated by antigen–antibody complexes, which has a potential to prevent complement-mediated cell damage. However, the effect of anti-complement therapy on AMR has not been well studied to date. Apheresis technology utilizing adsorption columns that are available in Europe and Asia may have a potential to reduce specific DSAs selectively.35,36 However, the efficacy and safety of these columns on AMR is unknown, and the availability and the high cost of development and production of the column in the United States may be significant limitations. Further investigations of these potential novel therapies for AMR are needed.
In summary, we report the efficacy of TPE followed by IVIG in reducing DSAs by HLA specificity in the treatment of AMR after kidney Txp. The treatment reduced DSA levels by 25.3 and 35.5% after three and six TPE procedures, respectively. However, the DSA reduction was much less marked after three TPE procedures. HLA Class I DSAs were reduced slightly more than Class II DSAs with least reduction rate of DSAs to DR51-53. Cr improvement rates were minimal with treatment. However, we found improvement of graft function for younger grafts and stabilization of older grafts for 6 months with AMR treatment. Our data also emphasize the importance of early initiation of treatment.
ACKNOWLEDGMENT
We thank Nathanael Bailey, MD, pathology fellow at the time of investigation at University of Michigan Health System for DSA data collection.
ABBREVIATIONS
- AMR
antibody-mediated rejection
- Cr
creatinine
- DSA(s)
donor-specific antibody(-ies)
- TPE
therapeutic plasma exchange
- t-point
time point (when followed by a number)
- Txp
transplantation
Footnotes
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
REFERENCES
- 1.Zachary AA, Montgomery RA, Leffell MS. Factors associated with and predictive of persistence of donor-specific antibody after treatment with plasmapheresis and intravenous immunoglobulin. Hum Immunol. 2005;66:364–70. doi: 10.1016/j.humimm.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 2.Zachary AA, Montgomery RA, Ratner LE, et al. Specific and durable elimination of antibody to donor HLA antigens in renal-transplant patients. Transplantation. 2003;76:1519–25. doi: 10.1097/01.TP.0000090868.88895.E0. [DOI] [PubMed] [Google Scholar]
- 3.Ramos EJ, Pollinger HS, Stegall MD, et al. The effect of desensitization protocols on human splenic B-cell populations in vivo. Am J Transplant. 2007;7:402–7. doi: 10.1111/j.1600-6143.2006.01632.x. [DOI] [PubMed] [Google Scholar]
- 4.Dau PC. Increased antibody production in peripheral blood mononuclear cells after plasma exchange therapy in multiple sclerosis. J Neuroimmunol. 1995;62:197–200. doi: 10.1016/0165-5728(95)00121-4. [DOI] [PubMed] [Google Scholar]
- 5.Paglieroni T, Caggiano V, MacKenzie MR. Effects of plasmapheresis on peripheral blood mononuclear cell populations from patients with macroglobulinemia. J Clin Apher. 1987;3:202–8. doi: 10.1002/jca.2920030403. [DOI] [PubMed] [Google Scholar]
- 6.Sadeghi M, Daniel V, Wang H, et al. Plasmapheresis adjusts inflammatory responses in potential kidney transplant recipients. Transplantation. 2013;95:1021–9. doi: 10.1097/TP.0b013e318286191b. [DOI] [PubMed] [Google Scholar]
- 7.Mayer L, Stohl W, Cunningham-Rundles C. Feedback inhibition of B cell differentiation by monomeric immunoglobulin. Int Rev Immunol. 1989;5:189–95. doi: 10.3109/08830188909061986. [DOI] [PubMed] [Google Scholar]
- 8.Tawfik DS, Cowan KR, Walsh AM, et al. Exogenous immunoglobulin downregulates T-cell receptor signaling and cytokine production. Pediatr Allergy Immunol. 2012;23:88–95. doi: 10.1111/j.1399-3038.2010.01129.x. [DOI] [PubMed] [Google Scholar]
- 9.Chin C, Chen G, Sequeria F, et al. Clinical usefulness of a novel C1q assay to detect immunoglobulin G antibodies capable of fixing complement in sensitized pediatric heart transplant patients. J Heart Lung Transplant. 2011;30:158–63. doi: 10.1016/j.healun.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland SM, Chen G, Sequeira FA, et al. Complement-fixing donor-specific antibodies identified by a novel C1q assay are associated with allograft loss. Pediatr Transplant. 2012;16:12–7. doi: 10.1111/j.1399-3046.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- 11.Tyan DB. New approaches for detecting complement-fixing antibodies. Curr Opin Organ Transplant. 2012;17:409–15. doi: 10.1097/MOT.0b013e328355fb9b. [DOI] [PubMed] [Google Scholar]
- 12.Tait BD, Susal C, Gebel HM, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013;95:19–47. doi: 10.1097/TP.0b013e31827a19cc. [DOI] [PubMed] [Google Scholar]
- 13.Zwirner NW, Dole K, Stastny P. Differential surface expression of MICA by endothelial cells, fibroblasts, keratinocytes, and monocytes. Hum Immunol. 1999;60:323–30. doi: 10.1016/s0198-8859(98)00128-1. [DOI] [PubMed] [Google Scholar]
- 14.Angaswamy N, Tiriveedhi V, Sarma NJ, et al. Interplay between immune responses to HLA and non-HLA self-antigens in allograft rejection. Hum Immunol. 2013;74:1478–85. doi: 10.1016/j.humimm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang M, Lu FM, Qu LX, et al. Serum major-histocompatibility-complex class I-related chain A antibody detection for the evaluation of graft dysfunction in renal allograft recipients. Chin Med J (Engl) 2011;124:2127–31. [PubMed] [Google Scholar]
- 16.Banasik M, Boratynska M, Koscielska-Kasprzak K, et al. Long-term follow-up of non-HLA and anti-HLA antibodies: incidence and importance in renal transplantation. Transplant Proc. 2013;45:1462–5. doi: 10.1016/j.transproceed.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 17.Qin Z, Lavingia B, Zou Y, et al. Antibodies against nucleolin in recipients of organ transplants. Transplantation. 2011;92:829–35. doi: 10.1097/TP.0b013e31822d0977. [DOI] [PubMed] [Google Scholar]
- 18.Mitate E, Kawano S, Nakao Y, et al. Concurrence of auto-antibodies to both laminin gamma1 and gamma2 subunits in a patient with kidney rejection response. Acta Derm Venereol. 2013;93:114–5. doi: 10.2340/00015555-1395. [DOI] [PubMed] [Google Scholar]
- 19.Iyer HS, Jackson AM, Zachary AA, et al. Transplanting the highly sensitized patient: trials and tribulations. Curr Opin Nephrol Hypertens. 2013;22:681–8. doi: 10.1097/MNH.0b013e328365b3b9. [DOI] [PubMed] [Google Scholar]
- 20.Kaposztas Z, Podder H, Mauiyyedi S, et al. Impact of rituximab therapy for treatment of acute humoral rejection. Clin Transplant. 2009;23:63–73. doi: 10.1111/j.1399-0012.2008.00902.x. [DOI] [PubMed] [Google Scholar]
- 21.Tzvetanov I, Spaggiari M, Joseph J, et al. The use of bortezomib as a rescue treatment for acute antibody-mediated rejection: report of three cases and review of literature. Transplant Proc. 2012;44:2971–5. doi: 10.1016/j.transproceed.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 22.Everly MJ, Everly JJ, Susskind B, et al. Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation. 2008;86:1754–61. doi: 10.1097/TP.0b013e318190af83. [DOI] [PubMed] [Google Scholar]
- 23.Walsh RC, Everly JJ, Brailey P, et al. Proteasome inhibitor-based primary therapy for antibody-mediated renal allograft rejection. Transplantation. 2010;89:277–84. doi: 10.1097/TP.0b013e3181c6ff8d. [DOI] [PubMed] [Google Scholar]
- 24.Walsh RC, Alloway RR, Girnita AL, et al. Proteasome inhibitor-based therapy for antibody-mediated rejection. Kidney Int. 2012;81:1067–74. doi: 10.1038/ki.2011.502. [DOI] [PubMed] [Google Scholar]
- 25.Hao WJ, Zong HT, Cui YS, et al. The efficacy and safety of alemtuzumab and daclizumab versus antithymocyte globulin during organ transplantation: a meta-analysis. Transplant Proc. 2012;44:2955–60. doi: 10.1016/j.transproceed.2012.05.085. [DOI] [PubMed] [Google Scholar]
- 26.Saghafi H, Rahbar K, Nobakht Haghighi A, et al. Efficacy of anti-interleukin-2 receptor antibody (daclizumab) in reducing the incidence of acute rejection after renal transplantation. Nephrourol Mon. 2012;4:475–7. doi: 10.5812/numonthly.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atlani M, Sharma RK, Gupta A. Basiliximab induction in renal transplantation: long-term outcome. Saudi J Kidney Dis Transpl. 2013;24:473–9. doi: 10.4103/1319-2442.111010. [DOI] [PubMed] [Google Scholar]
- 28.Ponticelli C. Basiliximab: efficacy and safety evaluation in kidney transplantation. Expert Opin Drug Saf. 2014;13:373–81. doi: 10.1517/14740338.2014.861816. [DOI] [PubMed] [Google Scholar]
- 29.van den Hoogen MW, Hesselink DA, van Son WJ, et al. Treatment of steroid-resistant acute renal allograft rejection with alemtuzumab. Am J Transplant. 2013;13:192–6. doi: 10.1111/j.1600-6143.2012.04328.x. [DOI] [PubMed] [Google Scholar]
- 30.Stewart ZA, Collins TE, Schlueter AJ, et al. Case report: eculizumab rescue of severe accelerated antibody-mediated rejection after ABO-incompatible kidney transplant. Transplant Proc. 2012;44:3033–6. doi: 10.1016/j.transproceed.2012.03.053. [DOI] [PubMed] [Google Scholar]
- 31.Goh BK, Chedid MF, Gloor JM, et al. The impact of terminal complement blockade on the efficacy of induction with polyclonal rabbit antithymocyte globulin in living donor renal allografts. Transpl Immunol. 2012;27:95–100. doi: 10.1016/j.trim.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Stegall MD, Chedid MF, Cornell LD. The role of complement in antibody-mediated rejection in kidney transplantation. Nat Rev Nephrol. 2012;8:670–8. doi: 10.1038/nrneph.2012.212. [DOI] [PubMed] [Google Scholar]
- 33.Heeger PS. A complementary approach to treating antibody-mediated transplant rejection. Kidney Int. 2010;78:125–7. doi: 10.1038/ki.2010.80. [DOI] [PubMed] [Google Scholar]
- 34.Poirier N, Blancho G. Recombinant human C1-inhibitor inhibits cytotoxicity induced by allo- and xenoantibodies. Transplant Proc. 2008;40:581–3. doi: 10.1016/j.transproceed.2008.01.045. [DOI] [PubMed] [Google Scholar]
- 35.Bohmig GA, Wahrmann M, Regele H, et al. Immunoadsorption in severe C4d-positive acute kidney allograft rejection: a randomized controlled trial. Am J Transplant. 2007;7:117–21. doi: 10.1111/j.1600-6143.2006.01613.x. [DOI] [PubMed] [Google Scholar]
- 36.Genberg H, Kumlien G, Wennberg L, et al. The efficacy of antigen-specific immunoadsorption and rebound of anti-A/B antibodies in ABO-incompatible kidney transplantation. Nephrol Dial Transplant. 2011;26:2394–400. doi: 10.1093/ndt/gfr237. [DOI] [PubMed] [Google Scholar]