Figure. Proportion of participants with HIV drug resistance at baseline and at treatment failure.
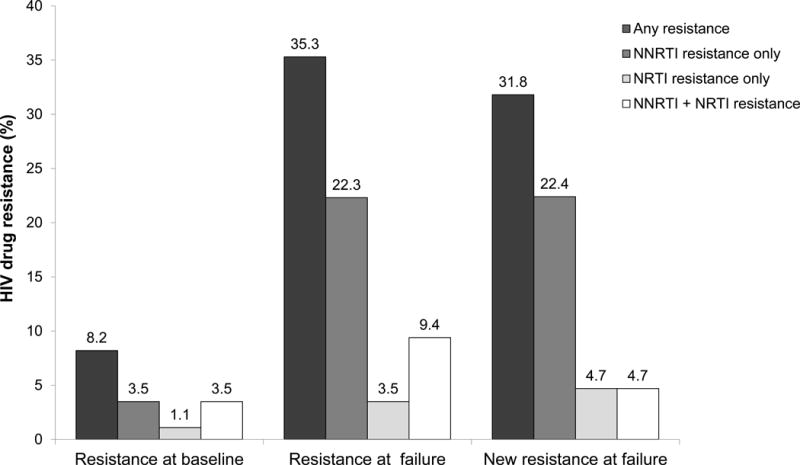
The figure shows the frequency of HIV drug resistance among participants in the early ART arm of HPTN 052 who failed ART (N=85 with paired baseline/failure results). Seven (8.2%) participants had resistance at baseline. The most common NNRTI and NRTI mutations were Y181C and M184V, which were each detected in three of the seven cases. All of the mutations detected at baseline, with the exception of one Y181C mutation, were also present in the corresponding failure samples. Thirty (35.3%) had resistance at ART failure. The most common NNRTI mutation was K103N, which was detected in 20 (66.7%) of the 30 cases. The most common NRTI mutation was M184V, which was detected in 11 (36.7%) of the 30 cases. Twenty-seven (90.0%) of those 30 participants had one or more resistance mutations detected at failure that was not present at the time of ART initiation (K103N [n=18], M184V [n=8], V106M [n=4), V108I [n=2], K238T [n=2), G190A [n=1), and K101E [n=1]). Twenty-three (85.2%) had new resistance to efavirenz, and most had cross-resistance to other NNRTIs (nevirapine [N=21], rilpivirine [N=2], and etravirine [N=1]). Eight (29.6%) had new NRTI resistance; all had new resistance to lamivudine and emtricitabine; one also had new resistance to abacavir and didanosine, due to the acquisition of M184V in addition to the baseline mutations M41L and T215E. Protease inhibitor resistance was not observed in any samples. Among the 78 (91.8%) participants who did not have baseline resistance, 23 (27.1%) had resistance at failure (not shown).
Abbreviations: NNRTI: non-nucleoside reverse transcriptase inhibitor; NRTI: nucleoside/nucleotide reverse transcriptase inhibitor.
