Abstract
Objectives
We evaluated whether differences in cardiovascular risk factors, as assessed by the Framingham risk scores for stroke and cardiovascular disease (FSRS and FCRS), contributed to disparities in all-cause mortality across race and regional strata of the United States.
Design
Race-region-specific FSRS and FCRS scores were computed for 30, 086 REGARDS participants who were recruited between January 2003 and October 2007. They were divided across 6 regions of the “Eight Americas” and then compared after adjusting for race and sex. Kaplan-Meier curves and hazard ratios for all-cause mortality were estimated between regions, first adjusted for age and sex, and then for the risk scores.
Results
After adjustment for age, sex, FCRS, and FSRS, there was no difference in mortality among Middle America Whites vs. Low Income White. However, mortality was lower among Middle America Blacks (-23%; p=0.06) and High Risk Urban Blacks (-24%; p=0.01) compared to Southern Low Income Rural Blacks. Compared to Middle American Whites, mortality was higher among Middle America Blacks (+39%; p<0.001), High Risk Urban Blacks (+35%; p<0.001) and Southern Low Income Rural Blacks (+85%; p<0.001).
Conclusion
Accounting for cardiovascular risk unmasked a greater disparity in mortality between Blacks and Whites; and among Southern Rural Blacks compared to Middle America Blacks and High Risk Urban Blacks.
Keywords: race, geography, cardiovascular risk, mortality
Introduction
In 1985, Secretary of the Department of Health and Human Services (DHHS) issued a landmark report which revealed large and persistent gaps in health status among Americans of different racial and ethnic groups that served as an impetus for addressing health inequalities for racial and ethnic minorities in the United States (US) (DHHS 1985). This report led to the establishment of the Office of Minority Health, with a mission to address these disparities within the nation.
Efforts to understand health disparities in the US have shown consistent and substantial gaps in mortality by race, particularly between Black and White Americans. Inequalities in access to healthcare, insurance, social status, income, and education, across race groups have been investigated extensively (Krieger et al. 2005; Barnett, Armstrong, and Casper 1999; Lemelin et al. 2009; Howard et al. 2000; Carnethon et al. 2006; Smith et al. 1997; Davey Smith et al. 1998; Sorlie et al. 1992; Geronimus et al. 1996; Mansfield et al. 1999). However disparities in life expectancy across race groups remain. In 2011, life expectancy at birth for Whites was significantly higher than for Blacks (79.0 vs. 75.3 years) ranging from 81.1 years for women (81.3 Whites vs. 78.2 years Blacks) to 76.3 years for men (76.6 Whites vs. 72.1 years Blacks) (Donna L. Hoyert 2012). Heart disease and cancer continue to be the leading causes of death in across race groups, accounting for 42 to 47% of all deaths (Donna L. Hoyert 2012; Heron 2015) An understanding of the mortality disparities is needed to formulate and implement effective interventions, programs and policies aimed at mitigating this recalcitrant issue.
Even larger disparities in life-expectancy exist by county of residence or a combination of race and county of residence (race-county) (Ezzati et al. 2002; Murray et al. 2006; Ezzati et al. 2008; Danaei et al. 2010). In the seminal work by Murray et al, the authors highlighted that the gap between the highest and lowest life expectancies for race-county combinations in the US is over 35 years by dividing the race-county regions into eight separate groups called the “Eight Americas” (Murray et al. 2006). The disparities in life expectancy cannot be explained by race, income, or basic health-care access and utilization alone. Therefore we hypothesized that a substantial proportion of the differences in mortality between the Eight-Americas would be attenuated by adjustment for cardiovascular disease (CVD) risk factors.
We aimed to evaluate if differences in cardiovascular risk factor burden, as assessed by the Framingham Stroke Risk Score (FSRS) and the Framingham Coronary Risk Score (FCRS), contributed to the these race-county disparities in mortality. To this end we implemented the “Eight Americas” definition of race-county within a large national, biracial prospective cohort -the REasons for Geographic And Racial Differences in Stroke (REGARDS) study and examined the role of CVD risk burden in differences in mortality among these groups. As the striking racial differences in stroke mortality are captured by contrasting blacks to whites (i.e., the stroke mortality of Asian/Pacific Islanders, Native American/Alaska Natives, and Hispanics [regardless of race] are similar to whites), recruitment to REGARDS was restricted to non-Hispanic white and black participants. With this restriction, “Asian” and “Western Native American” identified by Murray,(Murray et al. 2006) could not be included in these analyses. Also, only five among the 6 regions had sufficient representation to allow assessment of the distribution of CVD risk factors, FSRS and FCRS, and all-cause mortality. This assessment did not include Northern low income rural Whites due to their limited (n=136) participation.
Methods
Study cohort
The REasons for Geographic and Racial Differences in Stroke (REGARDS) study includes a population-based sample of US Black and White adults 45 years or older from the 48 contiguous US states. Study recruitment and data collection have been described previously (Howard et al. 2005). Briefly, between January 2003 and October 2007, eligible participants were identified from a commercially available list of residents and recruited through initial mail followed by telephone contact; only those participants who completed the initial telephone survey followed by an in-home visit, and agreed to subsequent telephone follow-up for changes in health status were enrolled in the study. The response (33%) and cooperation rates (49%) were similar to the rates reported in other epidemiologic studies (Jackson et al. 1996; MESA 2004).
By design, 56% of the sample was recruited from Southern US states commonly referred to as the “stroke buckle” (coastal North Carolina, South Carolina, and Georgia) and “stroke belt” (Alabama, Mississippi, Tennessee, Arkansas, Louisiana, and the remainder of North Carolina, South Carolina, and Georgia), and the rest recruited from the remaining 40 contiguous US states. Only those individuals who self-identified as non-Hispanic Black or White were eligible. The final REGARDS study population of 30,239 participants was comprised of 41% Blacks and 55% women. The REGARDS study protocol was approved by the Institutional Review Boards governing research in human subjects at the collaborating centers and all participants provided informed consent.
Data collection
Trained personnel conducted computer-assisted telephone interviews and subsequent in-home study visits during which they collected information on socio-demographic, lifestyle, and clinical factors, medication use, samples for laboratory assessment, anthropometric measurements, and electrocardiogram (ECG). CVD related information included blood pressure and lipid measures, self-reported diagnosis of myocardial infarction (MI) or stroke, and history of cardiovascular procedures (endarterectomy, aortic aneurysm repair, coronary artery bypass surgery, peripheral vascular surgery, and percutaneous transluminal coronary angioplasty).
Hypertension was categorized based on the Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) and defined as systolic blood pressure (SBP) ≥ 140 mm Hg or diastolic blood pressure (DBP) ≥ 90 mm Hg or current pharmacological treatment for hypertension (Chobanian et al. 2003). Blood pressure categorizations were made regardless of the use of antihypertensive medication and the higher category was selected when SBP and DBP fell into separate categories. Total cholesterol, high density lipoprotein (HDL) cholesterol, triglycerides, and glucose were measured by colorimetric reflectance spectrophotometry using the Ortho Vitros Clinical Chemistry System 950IRC instrument (Johnson & Johnson Clinical Diagnostics). Diabetes mellitus (DM) was defined as fasting glucose level ≥126 mg/dL (or non-fasting glucose level ≥200 mg/dL for those who failed to fast) or current pharmacological treatment (insulin or oral hypoglycemic) for DM.
Left ventricular hypertrophy (LVH) was defined using the electrocardiographic Sokolow-Lyon criteria (Sokolow and Lyon 1949). For this report, atrial fibrillation (AF) was diagnosed based on centrally-read ECG or self-report of physician-diagnosis. The present analysis was limited to participants without a history of CVD, defined as evidence of prior MI on ECG or by self-report, stroke or self-reported history of any of the cardiovascular procedures mentioned earlier.
The Framingham Stroke Risk Score was computed using age, SBP, DM, smoking, AF, LVH and use of hypertensive medication (Wolf et al. 1991; D'Agostino et al. 1994). Age and systolic blood pressure were classified at every 10-unit change whereas the remaining variables were dichotomized with 1 if yes and 0 if no. The Framingham Coronary Risk Score was calculated based on age (10-unit change, sex, DM (yes/no), smoking (yes/no), JNC-7 blood pressure categories (Chobanian et al. 2003) National Cholesterol Education Program (NCEP) total cholesterol categories, and HDL cholesterol categories (Wilson et al. 1998; ATPII 1993).
Population Subgroups
A relatively large part of the overall observed race–county life expectancy disparities were previously explained by grouping the US population by race and county characteristics (Murray et al. 2006; Ezzati et al. 2008). Based on these findings, we implemented the “Eight Americas” sub-grouping of the US race–county regions as our unit of analysis within the REGARDS cohort. Eight-Americas includes Asian, Northland Low Income Rural White, Middle America White, Low income Whites in Appalachia and the Mississippi Valley, Western Native American, Black Middle America, Southern Low Income rural Black, and High Risk Urban Black regions. Our analysis excluded the Asian, Western Native Americans and Northern Low Income Rural Whites.
Outcome
All-cause mortality
Participants or their proxies were contacted every six months by telephone to obtain information on outcomes of interest or death during the previous six months. For proxy reported deaths, interview was conducted with next of kin. Follow-up for the current analysis was available through March 31, 2013. For suspected deaths, date of death was confirmed through the Social Security Death Index, death certificates, or the National Death Index. Follow-up time was calculated as the number of days from the baseline in-home visit to date of death or the last REGARDS study telephone contact prior to March 31, 2013, if not deceased.
Statistical analysis
Baseline characteristics were described as mean (standard deviation) for continuous variables and proportions for categorical variables. Analysis of variance was used to assess whether age-sex adjusted differences for cardiovascular risk profiles (FSRS and FCRS) differed between the 6 regions.
Differences in FCRS and FSRS across the 6 regions were estimated using a general linear model with adjustment for age and sex. Differences in individual risk factors that comprise the risk scores were also evaluated. Crude differences in all cause-mortality were estimated using Kaplan-Meier estimates and hazard ratios estimated using cox proportional hazards analysis with: 1) adjustment for age and sex, and 2) after further adjustment for the Framingham risk scores. The analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC) with two-tailed p-values determined at significance level of 0.05.
Results
Of the 30,239 participants, 56 were excluded due to data anomalies, and 97 due to geocoding issues, resulting in an analysis cohort of 30,086 participants. The 30,086 REGARDS participants were distributed across Northland Low Income Rural White (0.45%), Middle America White (51.4%), Low income White in Appalachia and the Mississippi valley (6.7%, Figure 1A), Black Middle America (22.7%), Southern Low Income Rural Black (9.2%) and High Risk Urban Black (9.6%, Figure 1B). Tables 1 and 2 present the distribution of socio-demographic characteristics and cardiovascular risk factors across the race-county regions.
Figure 1.
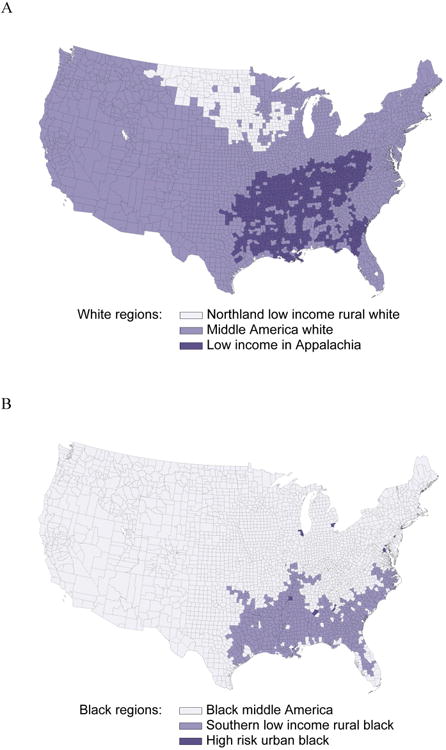
Distribution of REGARDS participants by race (A: Whites; B: Blacks) and county of residence.
Table 1.
Demographic characteristics of REGARDS study participants (N=30,086)a stratified by race-county regions.
| All | US region as defined by Murray et al | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| White Regions | Black Regions | ||||||
|
|
|||||||
| Characteristics | North-land White | Middle America White | Low Income White | Middle America Black | Southern Low Income Rural Blacks | High Risk Urban Black | |
|
| |||||||
| Total participants | 30086 | 136 | 15464 | 2020 | 6824 | 2761 | 2881 |
| Percent | |||||||
|
| |||||||
| Blacks | 41.4 | 0.0 | 0.0 | 0.0 | 100.0 | 100.0 | 100.0 |
| Female | 55.1 | 53.7 | 49.9 | 51.9 | 60.8 | 65.4 | 61.8 |
| Income | |||||||
| ≤$20k | 18.1 | 14.0 | 11.2 | 17.5 | 24.3 | 34.2 | 26.0 |
| $20k-$34k | 24.2 | 22.8 | 22.1 | 26.8 | 26.6 | 26.0 | 26.6 |
| $35k-$74k | 29.6 | 42.6 | 32.9 | 30.2 | 26.6 | 21.2 | 26.0 |
| ≥$75k | 15.8 | 11.0 | 21.6 | 14.0 | 9.8 | 5.8 | 9.6 |
| Refused | 12.3 | 9.6 | 12.2 | 11.5 | 12.7 | 12.8 | 11.9 |
| Education | |||||||
| ≥College | 34.7 | 22.8 | 42.8 | 29.8 | 26.5 | 23.0 | 25.8 |
| Some College | 26.8 | 34.6 | 27.1 | 26.1 | 28.4 | 21.0 | 27.6 |
| High school | 25.9 | 36.0 | 23.4 | 31.9 | 27.1 | 30.5 | 27.4 |
| ≤High school | 12.6 | 6.6 | 6.7 | 12.2 | 18.1 | 25.5 | 19.1 |
| Self-reported general health | |||||||
| Excellent | 16.0 | 16.9 | 20.8 | 17.3 | 10.1 | 8.3 | 10.4 |
| Very good | 30.5 | 43.4 | 35.9 | 31.4 | 24.6 | 20.4 | 23.5 |
| Good | 35.1 | 31.6 | 31.0 | 33.1 | 40.1 | 40.7 | 41.4 |
| Fair | 15.0 | 7.4 | 9.7 | 13.8 | 20.9 | 25.2 | 20.4 |
| Poor | 3.5 | 0.7 | 2.7 | 4.4 | 4.2 | 5.4 | 4.2 |
Of the 30,239 participants, 56 were excluded due to data anomalies, and 97 excluded due to geocoding issues, resulting in the analysis cohort of 30,086 participants.
Table 2.
Clinical characteristics of REGARDS study participants (N=30,086)a stratified by race-county regions.
| All | US region as defined by Murray et al | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||
| White Regions | Black Regions | |||||||||||||
|
|
||||||||||||||
| Characteristics | North-land White | Middle America White | Low Income White | Middle America Black | Southern Low Income Rural Blacks | High Risk Urban Black | ||||||||
| Cardiovascular Risk Factors | Percent | |||||||||||||
|
| ||||||||||||||
| Smokers | 14.6 | 9.6 | 12.3 | 15.0 | 17.0 | 16.7 | 19.4 | |||||||
| Hypertensive | 59.2 | 53.7 | 50.1 | 54.9 | 70.3 | 74.4 | 70.6 | |||||||
| Taking Antihypertensive medications | 53.6 | 42.1 | 44.5 | 49.1 | 65.1 | 69.5 | 63.4 | |||||||
| Diabetes | 22.0 | 15.2 | 15.5 | 18.3 | 30.2 | 34.3 | 28.9 | |||||||
| Left Ventricular Hypertrophy | 9.9 | 7.0 | 6.5 | 6.7 | 14.5 | 14.8 | 15.2 | |||||||
| Atrial Fibrillation | 8.8 | 9.0 | 9.6 | 9.2 | 7.5 | 8.5 | 7.5 | |||||||
| Heart Disease | 17.9 | 19.7 | 19.2 | 22.5 | 15.2 | 15.4 | 16.3 | |||||||
|
| ||||||||||||||
| Cardiovascular Risk Factors | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
|
| ||||||||||||||
| Age | 64.8 | 9.4 | 66.1 | 10.3 | 65.5 | 9.5 | 64.4 | 8.9 | 64.4 | 9.3 | 62.6 | 9.2 | 61.8 | 9.2 |
| Total Cholesterol | 192.1 | 40.1 | 190.2 | 38.1 | 191.1 | 39.0 | 194.0 | 42.7 | 193.5 | 41.1 | 191.9 | 41.3 | 192.7 | 41.2 |
| High Density Lipoprotein | 51.8 | 16.2 | 49.9 | 15.5 | 50.9 | 16.2 | 48.6 | 15.9 | 53.7 | 16.1 | 52.6 | 15.2 | 53.8 | 16.4 |
| Systolic Blood Pressure | 127.6 | 16.7 | 125.5 | 16.9 | 125.2 | 15.8 | 126.5 | 15.6 | 130.1 | 17.0 | 130.8 | 17.5 | 132.3 | 18.2 |
| Diastolic Blood Pressure | 76.5 | 9.7 | 75.1 | 9.1 | 75.1 | 9.2 | 75.6 | 9.1 | 78.4 | 9.9 | 78.6 | 10.0 | 78.5 | 10.4 |
| Framingham Stroke Score | 10.0 | 10.6 | 10.1 | 10.9 | 9.4 | 10.0 | 9.3 | 9.9 | 10.8 | 11.2 | 10.4 | 11.3 | 11.4 | 11.7 |
| Framingham CHD Score | 9.8 | 9.5 | 9.7 | 9.4 | 9.4 | 9.0 | 10.0 | 9.8 | 10.3 | 10.0 | 9.8 | 9.8 | 10.8 | 9.3 |
SD: standard deviation
Of the 30,239 participants, 56 were excluded due to data anomalies, and 97 excluded due to geocoding issues, resulting in the analysis cohort of 30,086 participants.
Whites residing in Low Income White areas had higher FCRS (p<0.001) and FSRS (p<0.01) compared to Middle America Whites (Table 3) explained by higher SBP (p<0.001), DBP (p=0.05), total cholesterol (p=0.04) and HDL levels (p<0.001). Low income Whites were more likely to smoke (p<0.01), have DM (p<0.001), CHD (p<0.001), and hypertension (p<0.001) and to use antihypertensive medications (p<0.001).
Table 3.
Age and sex adjusted Framingham CHD and stroke scores among whitesa (N=17, 525) and blacks (N=12,511) as well as the differences in risk factors according to race-county regions.
| Risk factor/Score | Middle America White | Low Income White | Southern Low Income Rural Black | Middle America Black | High Risk Urban Black | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |||
| Framingham CHD score | 9.65 | 0.07 | 10.79 | 0.20 | 11.26 | 0.19 | 10.99 | 0.12 | 11.55 | 0.19 | ||
| Framingham Stroke Score | 9.35 | 0.07 | 9.90 | 0.19 | 11.60 | 0.20 | 10.76 | 0.13 | 11.14 | 0.20 | ||
| Systolic Blood Pressure (mmHg) | 125.20 | 0.12 | 126.90 | 0.34 | 131.40 | 0.33 | 130.30 | 0.21 | 132.40 | 0.32 | ||
| Diastolic Blood Pressure (mmHg) | 75.10 | 0.07 | 75.50 | 0.20 | 78.60 | 0.19 | 78.60 | 0.12 | 78.80 | 0.19 | ||
| Total Cholesterol | 191.30 | 0.31 | 193.2 | 0.86 | 189.70 | 0.80 | 192.20 | 0.51 | 191.20 | 0.78 | ||
| High Density Lipoprotein | 51.00 | 0.12 | 48.40 | 0.34 | 51.40 | 0.30 | 52.70 | 0.19 | 52.70 | 0.30 | ||
|
| ||||||||||||
| OR | OR | 95% CI | OR | OR | 95% CI | OR | 95% CI | |||||
|
| ||||||||||||
| Hypertension | 1.00b | 1.29 | 1.17, 1.41 | 1.00 | 0.77 | 0.70, 0.85 | 0.77 | 0.69, 0.87 | ||||
| Use of antihypertensive | 1.00 | 1.27 | 1.15, 1.40 | 1.00 | 0.78 | 0.71, 0.86 | 0.72 | 0.64, 0.80 | ||||
| Diabetes mellitus | 1.00 | 1.25 | 1.11, 1.42 | 1.00 | 0.80 | 0.73, 0.89 | 0.75 | 0.67, 0.84 | ||||
| Current Smoking | 1.00 | 1.21 | 1.05, 1.38 | 1.00 | 1.10 | 0.97, 1.24 | 1.33 | 1.16, 1.53 | ||||
| Atrial Fibrillation | 1.00 | 1.02 | 0.87, 1.20 | 1.00 | 0.88 | 0.74, 1.03 | 0.87 | 0.71, 1.05 | ||||
| Left Ventricular Hypertrophy | 1.00 | 1.08 | 0.90, 1.31 | 1.00 | 0.92 | 0.81, 1.05 | 0.98 | 0.84, 1.13 | ||||
| History of heart disease | 1.00 | 1.35 | 1.20, 1.52 | 1.00 | 0.90 | 0.80, 1.02 | 0.99 | 0.86, 1.14 | ||||
SE: standard error, OR: odds ratio, CI: confidence intervals
Northland Whites (n=136) excluded as they represented only 0.45% of the REGARDS cohort
An odds ratio of 1.00 denotes null association and is considered a reference. Among Whites, the Middle America Whites serve as the reference groups whereas among Blacks, the Southern Low Income Rural Black serves as the reference group.
The FCRS for High Risk Urban Blacks were higher than those for Blacks in Middle America (p=0.01) but no significant differences were observed between Southern Low Income Rural Blacks and High Risk Urban Blacks (p=0.24) or Blacks in Middle America (p=0.28; Table 3). The FSRS for Southern Low Income Rural Blacks were higher than those for Middle America Blacks (p<0.001) and marginally (p=0.09) higher than those in High Risk Urban Blacks but no significant differences were observed between Middle America Blacks and High Risk Urban Blacks (p=0.11).
Unlike the risk distribution among Whites, the prevalence of risk factors was not uniformly high (or low) in one geographic Black subgroup. SBP was highest among High Risk Urban Blacks (p<0.001 compared to Middle America Blacks; p=0.04 compared to Southern Low Income Rural Blacks). Total cholesterol was highest in Middle America Blacks (p=0.01 compared to Southern Low Income Rural Blacks) while HDL was lowest among Southern Low Income Rural Blacks (p<0.001 compared to Middle America Blacks; p>0.01 compared to High Risk Urban Blacks; Table 3).
Compared to Southern Low Income Rural Blacks, High Risk Urban Blacks and Middle America Blacks had lower likelihood of diabetes (both p<0.001), hypertension (p<0.001) and reported lower use of antihypertensive medications (p<0.001). Only smoking was more prevalent among High Risk Urban Blacks (p<0.01 compared to Middle America Blacks, p<0.001 compared to Southern Low Income Rural Blacks; Table 3).
Although not statistically significant, Low Income Whites had a 12% higher mortality compared to Middle America Whites after adjusting for age and sex (Table 4) and a 7% higher mortality after further adjusting for FCRS and FSRS (Figure 2a). Whereas mortality was lower among Middle America Blacks (13% lower; p=0.04) and High Risk Urban Blacks (9% lower; p= 0.24) compared to Southern Low Income Rural Blacks. There was no difference in mortality between Blacks in High Risk Urban areas and those in Middle America. Further adjusting for FCRS and FSRS, Middle America Blacks had 23% (p=0.06) lower mortality and High Risk Urban Blacks had a 24% (p=0.01; Figure 2b) lower mortality compared to Southern Low Income Rural Blacks.
Table 4.
Age and sex adjusted mortality among whites and blacks REGARDS participants by race-county regions.
| Stratified by race | Both races and middle America white as reference | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| Deaths | Mortality Ratea | After adjustment for age and sex | After further adjustment for Framingham Stroke and CHD risk scores | After adjustment for age and sex | After further adjustment for Framingham Stroke and CHD risk scores | ||||||||
|
|
|||||||||||||
| Total N | N | % | MR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Whitesb | |||||||||||||
|
|
|||||||||||||
| Low Income White | 2000 | 187 | 9.4 | 1861 | 1612, 2147 | 1.12 | 0.96, 1.31 | 1.07 | 0.85, 1.35 | 1.10 | 0.95, 1.28 | 1.05 | 0.83, 1.33 |
| Middle America White | 15320 | 1468 | 9.6 | 1905 | 1810, 2005 | 1.00c | 1.00 | 1.00 | 1.00 | ||||
|
|
|||||||||||||
| Blacks | |||||||||||||
|
|
|||||||||||||
| Middle America Black | 6689 | 746 | 11.2 | 2333 | 2172, 2507 | 0.87 | 0.75, 1.00 | 0.77 | 0.64, 0.93 | 1.45 | 1.32, 1.58 | 1.39 | 1.23, 1.58 |
| High Risk Urban Black | 2802 | 354 | 12.6 | 2557 | 2304, 2838 | 0.91 | 0.78, 1.07 | 0.76 | 0.61, 0.94 | 1.51 | 1.35, 1.70 | 1.35 | 1.14, 1.60 |
| Southern Low Income Rural Blacks | 2707 | 274 | 10.1 | 2268 | 2015, 2553 | 1.00 | 1.00 | 1.69 | 1.49, 1.93 | 1.83 | 1.54, 2.18 | ||
SE: standard error, MR: Mortality rate, HR: hazards ratio, CI: confidence intervals
Mortality rate was calculated per 100, 000 person-years.
Northland Whites (n=136) excluded as they represented only 0.45% of the REGARDS cohort. Asian and Western Native American as defined by Murray et al were not represented in the REGARDS cohort
A hazard ratio of 1.00 denotes null association and serves as a reference. Middle America White serves as a reference for the stratified analysis within Whites. Southern Low Income Rural Blacks serve as a reference for the stratified analysis within Blacks
Figure 2.
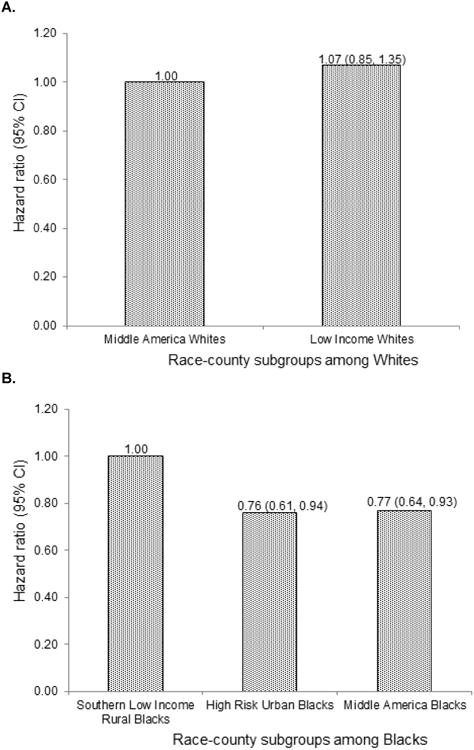
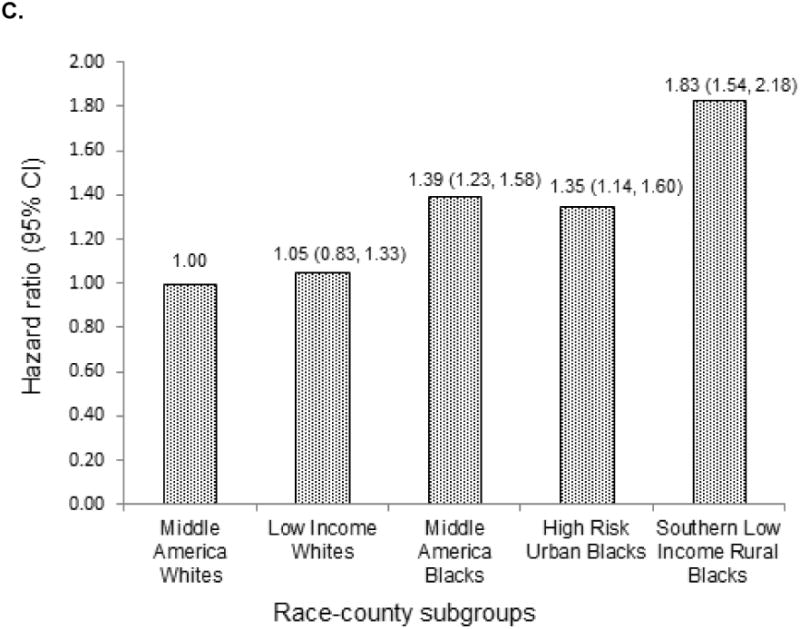
All-cause mortality among race-county residence a) among Blacks wherein Southern Low Income Rural Blacks serve as the reference group b) among Whites wherein Middle America Whites serve as the reference group c) across the race groups Blacks and Whites wherein Middle America Whites serve as the reference group.
Figure 3 demonstrates the higher incidence of mortality among Blacks compared to Whites. Compared to Middle America Whites, after adjusting for age, sex, FSRS and FCRS, mortality was 39% higher among Middle America Blacks (p<0.001), 35% higher among High Risk Urban Blacks (p<0.001) and 83% higher among Southern Low Income Rural Blacks (p<0.001, Figure 2c).
Figure 3.
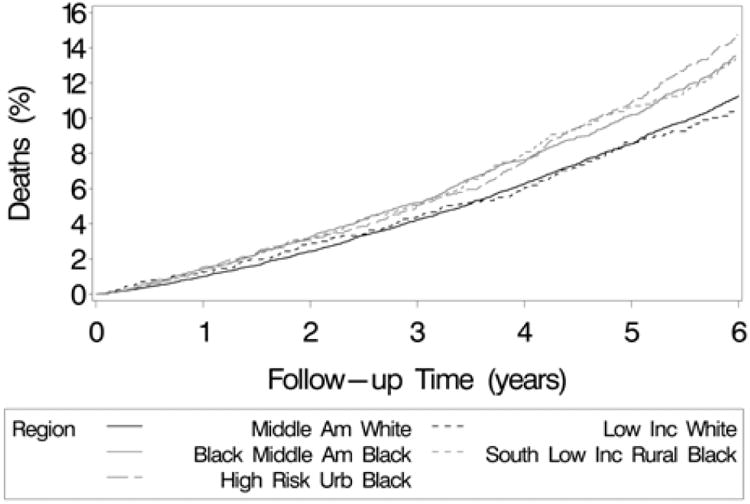
Cumulative incidence of all-cause mortality among REGARDS participants by race and county of residence.
Discussion
The disparities in life expectancies and mortality across racial and geographic subgroups have been attributed to factors related to socio-economics, demographics, the environment, health-related behavior and health care access (Cullen, Cummins, and Fuchs 2012). Our assessment of disparities in mortality in the population-based REGARDS cohort shows that despite the varied distribution of CVD risk factors, and FSRS and FCRS across race-county regions, mortality differences among White participants were not significantly influenced by CVD risk in our study. However among Blacks, accounting for CVD risk unmasked a greater disparity with Southern Low Income Rural Blacks experiencing higher mortality compared to the other two Black subgroups, and Whites.
The prevalence of hypertension and DM was higher among Southern Low Income Rural Blacks compared to the other two Black subgroups. Interventions to combat these modifiable risk factors could translate into gains in life-expectancy as recently demonstrated by Danaei et al (Danaei et al. 2010). Four modifiable risk factors –smoking, blood pressure, blood glucose, and adiposity – were associated with reduced life expectancy in both men and women. Our work identifies race-county regions for Whites and Blacks wherein targeted risk factor reduction could translate into reduced mortality.
Previous studies from REGARDS have focused broadly on regional differences in mortality across race groups. A recent study within REGARDS reported the association of FSRS with higher stroke risk (McClure et al. 2014). Herein, we demonstrate that the racial differences in mortality persist even after controlling for age, sex, and Framingham risk scores. This indicates that differential susceptibility or earlier accumulation of risk factors (higher cumulative risk) as well as other yet-to-be-identified/ undocumented risk factors may play an important role in disparities in mortality (Howard et al. 2011). Unmeasured factors are likely playing a major role leading to the highest mortality observed in Southern Low Income Rural Blacks. Of note, REGARDS only enrolled participants of age 45 years or older, thus the well-documented higher mortality attributable to violent deaths among younger Blacks did not contribute to our observations. Future efforts should focus on identifying additional risk factors and including them in assessing and addressing racial disparities.
The REGARDS cohort provides a unique opportunity to study the racial and geographic differences in CHD and stroke risk due to the higher proportion of Blacks and wide representation of other regions of the US. Additionally, REGARDS objectively measured factors constituting the FSRS and FCRS thus providing accurate, reliable and validated risk assessment compared to self-reported data used in national surveillance data sources. As REGARDS was designed to examine differences between Blacks and Whites, only five among the 6 regions had sufficient representation to allow assessment of the distribution of CVD risk factors, FSRS and FCRS, and all-cause mortality. Data from other race/ethnicities was not available and therefore assessment of Asian, Western Native American and Northern low income rural Whites as defined by Murray et al was not possible.
Several studies examining factors associated with health disparities have largely focused on individual or some composite measures of socio-economic and/or environmental factors(Adler and Rehkopf 2008). We implemented the 8-Americas method which is a composite of race, location of the county of residence, population density, race-specific county-level per capita income and cumulative homicide rate and provides broad and distinct race-county categories. While previous studies have focused on between race or race-sex differences (Cullen, Cummins, and Fuchs 2012; Adler and Rehkopf 2008), we also focus on within race differences, enabling the unmasking of a greater disparity in mortality among Southern Low Income Rural Blacks.
Intervention strategies tailored by racial/geographic regions are needed to improve the overall efficacy of already existing therapeutic interventions to mitigate and reduce risk for CVD and mortality. For example, as demonstrated by the differential distribution of risk factors in Table 1, the prevalence of hypertension and diabetes among Southern Low Income Rural Blacks compared to the other two Black subgroups was higher. Earlier studies from REGARDS suggest that higher prevalence of hypertension and diabetes increases the likelihood of other complicating comorbid conditions such as end-stage renal disease which in turn may increase the risk of mortality (Plantinga et al. 2013). Blacks were also more likely to have diets rich in cholesterol (Newby et al. 2012, 2011) and were less likely to be aggressively treated or controlled for hyperlipidemia despite equal health care utilization as Whites (Safford et al. 2015). More importantly, Black males were reported to be unaware of their hyperlipidemia (Safford et al. 2015) and are therefore less likely to receive or request treatment for it. Blacks also suffer a greater burden of pre-diabetes (Lee et al. 2014) and as a result may be at increased risk for early diabetes and unaware of the need for prevention or early treatment interventions. Therefore, interventions focused on curbing risk factors need to be targeted to and tailored for Blacks and specifically the Southern Low Income Rural Blacks.
The higher risk burden for stroke and CHD in the low income areas for both Blacks and Whites highlights that “All Blacks are not equal” and “Not all Whites are equal.” Efforts to better target culturally concordant interventions to improve mortality among Southern Low Income Blacks are urgently needed.
Key messages.
Our assessment of disparities in mortality in the population-based REGARDS cohort shows that despite the varied distribution of CVD risk factors, and FSRS and FCRS across race-county regions, mortality differences among White participants were not significantly influenced by CVD risk in our study.
However among Blacks, accounting for CVD risk unmasked a greater disparity with Southern Low Income Rural Blacks experiencing higher mortality compared to the other two Black subgroups, and Whites.
This highlights the need for interventions that are targeted and culturally concordant to improve mortality especially among Blacks, and more specifically among Southern Low Income Blacks.
Acknowledgments
Funding support: This REGARDS research project is supported by a cooperative agreement from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service (grant U01 NS041588). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Additional support was received from National Heart Lung and Blood Institute (grant RO1HL092173, NAL, R01HL080477, K24HL111154, MS). The authors thank the other investigators, and the staff of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Adler NE, Rehkopf DH. U.S. disparities in health: descriptions, causes, and mechanisms. Review of Annu Rev Public Health. 2008;29:235–52. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- ATPII. Summary of the second report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II) Review of JAMA. 1993;269(23):3015–23. [PubMed] [Google Scholar]
- Barnett E, Armstrong DL, Casper ML. Evidence of increasing coronary heart disease mortality among black men of lower social class. Review of Ann Epidemiol. 1999;9(8):464–71. doi: 10.1016/s1047-2797(99)00027-7. [DOI] [PubMed] [Google Scholar]
- Carnethon MR, Lynch EB, Dyer AR, Lloyd-Jones DM, Wang R, Garside DB, Greenland P. Comparison of risk factors for cardiovascular mortality in black and white adults. Review of Arch Intern Med. 2006;166(11):1196–202. doi: 10.1001/archinte.166.11.1196. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Review of Hypertension. 2003;42(6):1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Cullen MR, Cummins C, Fuchs VR. Geographic and racial variation in premature mortality in the U.S.: analyzing the disparities. Review of PLoS ONE. 2012;7(4):e32930. doi: 10.1371/journal.pone.0032930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Review of Stroke. 1994;25(1):40–3. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- Danaei G, Rimm EB, Oza S, Kulkarni SC, Murray CJ, Ezzati M. The promise of prevention: the effects of four preventable risk factors on national life expectancy and life expectancy disparities by race and county in the United States. Review of PLoS Med. 2010;7(3):e1000248. doi: 10.1371/journal.pmed.1000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey Smith G, Neaton JD, Wentworth D, Stamler R, Stamler J. Mortality differences between black and white men in the USA: contribution of income and other risk factors among men screened for the MRFIT. MRFIT Research Group. Multiple Risk Factor Intervention Trial. Review of Lancet. 1998;351(9107):934–9. doi: 10.1016/s0140-6736(00)80010-0. [DOI] [PubMed] [Google Scholar]
- DHHS. Executive Summary. I. HHS Office of Minority Health Resource Center; 1985. Report of the Secretary's Task Force on Black and Minority Health. 1985. [Google Scholar]
- Hoyert Donna L, PhD, Xu Jiaquan., MD Deaths: Preliminary Data for 2011. National Vital Statistics Reports: Division of Vital Statistics. 2012 [PubMed] [Google Scholar]
- Ezzati M, Friedman AB, Kulkarni SC, Murray CJ. The reversal of fortunes: trends in county mortality and cross-county mortality disparities in the United States. Review of PLoS Med. 2008;5(4):e66. doi: 10.1371/journal.pmed.0050066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ Group Comparative Risk Assessment Collaborating. Selected major risk factors and global and regional burden of disease. Review of Lancet. 2002;360(9343):1347–60. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- Geronimus AT, Bound J, Waidmann TA, Hillemeier MM, Burns PB. Excess mortality among blacks and whites in the United States. Review of N Engl J Med. 1996;335(21):1552–8. doi: 10.1056/NEJM199611213352102. [DOI] [PubMed] [Google Scholar]
- Heron M. Deaths: Leading Causes for 2011. Review of Natl Vital Stat Rep. 2015;64(7):1–96. [PubMed] [Google Scholar]
- Howard G, Anderson RT, Russell G, Howard VJ, Burke GL. Race, socioeconomic status, and cause-specific mortality. Review of Ann Epidemiol. 2000;10(4):214–23. doi: 10.1016/s1047-2797(00)00038-7. [DOI] [PubMed] [Google Scholar]
- Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Review of Neuroepidemiology. 2005;25(3):135–43. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, Cushman M, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Review of Ann Neurol. 2011;69(4):619–27. doi: 10.1002/ana.22385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R, Chambless LE, Yang K, Byrne T, Watson R, Folsom A, Shahar E, Kalsbeek W. Differences between respondents and nonrespondents in a multicenter community-based study vary by gender ethnicity. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Review of J Clin Epidemiol. 1996;49(12):1441–46. doi: 10.1016/0895-4356(95)00047-x. [DOI] [PubMed] [Google Scholar]
- Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Review of Am J Public Health. 2005;95(2):312–23. doi: 10.2105/AJPH.2003.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LT, Alexandrov AW, Howard VJ, Kabagambe EK, Hess MA, McLain RM, Safford MM, Howard G. Race, regionality and pre-diabetes in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Review of Prev Med. 2014;63:43–7. doi: 10.1016/j.ypmed.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemelin ET, Diez Roux AV, Franklin TG, Carnethon M, Lutsey PL, Ni H, O'Meara E, Shrager S. Life-course socioeconomic positions and subclinical atherosclerosis in the multi-ethnic study of atherosclerosis. Review of Soc Sci Med. 2009;68(3):444–51. doi: 10.1016/j.socscimed.2008.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield CJ, Wilson JL, Kobrinski EJ, Mitchell J. Premature mortality in the United States: the roles of geographic area, socioeconomic status, household type, and availability of medical care. Review of Am J Public Health. 1999;89(6):893–8. doi: 10.2105/ajph.89.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure LA, Kleindorfer DO, Kissela BM, Cushman M, Soliman EZ, Howard G. Assessing the performance of the Framingham Stroke Risk Score in the reasons for geographic and racial differences in stroke cohort. Review of Stroke. 2014;45(6):1716–20. doi: 10.1161/strokeaha.114.004915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MESA. MESA Exam 1 Participation Rate (10/13/2004) [Accessed 27 January];2012 http://www.mesa-nhlbi.org/participation.aspx.
- Murray CJ, Kulkarni SC, Michaud C, Tomijima N, Bulzacchelli MT, Iandiorio TJ, Ezzati M. Eight Americas: investigating mortality disparities across races, counties, and race-counties in the United States. Review of PLoS Med. 2006;3(9):e260. doi: 10.1371/journal.pmed.0030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby PK, Noel SE, Grant R, Judd S, Shikany JM, Ard J. Race and region are associated with nutrient intakes among black and white men in the United States. Review of J Nutr. 2011;141(2):296–303. doi: 10.3945/jn.110.130583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby PK, Noel SE, Grant R, Judd S, Shikany JM, Ard J. Race and region have independent and synergistic effects on dietary intakes in black and white women. Review of Nutr J. 2012;11:25. doi: 10.1186/1475-2891-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantinga LV, Howard J, Judd S, Muntner P, Tanner R, Rizk D, Lackland DT, Warnock DG, Howard G, McClellan WM. Association of duration of residence in the southeastern United States with chronic kidney disease may differ by race: the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort study. Review of Int J Health Geogr. 2013;12:17. doi: 10.1186/1476-072X-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safford MM, Gamboa CM, Durant RW, Brown TM, Glasser SP, Shikany JM, Zweifler RM, Howard G, Muntner P. Race-sex differences in the management of hyperlipidemia: the REasons for Geographic and Racial Differences in Stroke study. Review of Am J Prev Med. 2015;48(5):520–7. doi: 10.1016/j.amepre.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GD, Hart C, Blane D, Gillis C, Hawthorne V. Lifetime socioeconomic position and mortality: prospective observational study. Review of BMJ. 1997;314(7080):547–52. doi: 10.1136/bmj.314.7080.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Review of Am Heart J. 1949;37(2):161–86. doi: 10.1016/0002-8703(49)90562-1. [DOI] [PubMed] [Google Scholar]
- Sorlie P, Rogot E, Anderson R, Johnson NJ, Backlund E. Black-white mortality differences by family income. Review of Lancet. 1992;340(8815):346–50. doi: 10.1016/0140-6736(92)91413-3. [DOI] [PubMed] [Google Scholar]
- Wilson PWF, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Review of Circulation. 1998;97(18):1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Review of Stroke. 1991;22(3):312–8. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]


