Rheumatology key message
Catastrophic antiphospholipid syndrome is associated with excessive complement activation explaining the clinical efficacy of complement inhibitory treatment.
Sir, Research suggests that the complement system plays a role in the pathophysiology of APS, and experimental studies have revealed a requirement for C5 in APS-induced thrombus formation [1]. However, limited data are available on complement activation in the potentially life-threatening catastrophic form of APS (CAPS) [2]. Despite occasional reports of beneficial clinical effects of the complement inhibitor eculizumab in CAPS [3], the degree and pattern of complement activation has not been investigated. Such information is required as a rationale for therapeutic complement inhibition and is provided in the present report.
Plasma samples for measurement of complement activation products (C4bc for the classical and lectin pathways, C3bBbP for the alternative pathway, C3bc for the common pathway and sC5b-9 for the terminal pathway) were obtained in EDTA tubes, immediately placed on crushed ice, centrifuged and stored at −80°C. The activation product assays, based on specific monoclonal antibodies to neoepitopes selectively exposed in the activation products, have been described in detail previously [4]. Routine C3, C4, haematology and biochemical analyses were performed in the laboratory. Eculizumab was obtained from Alexion Pharmaceuticals (Lausanne, Switzerland). Written informed consent was obtained from the patient.
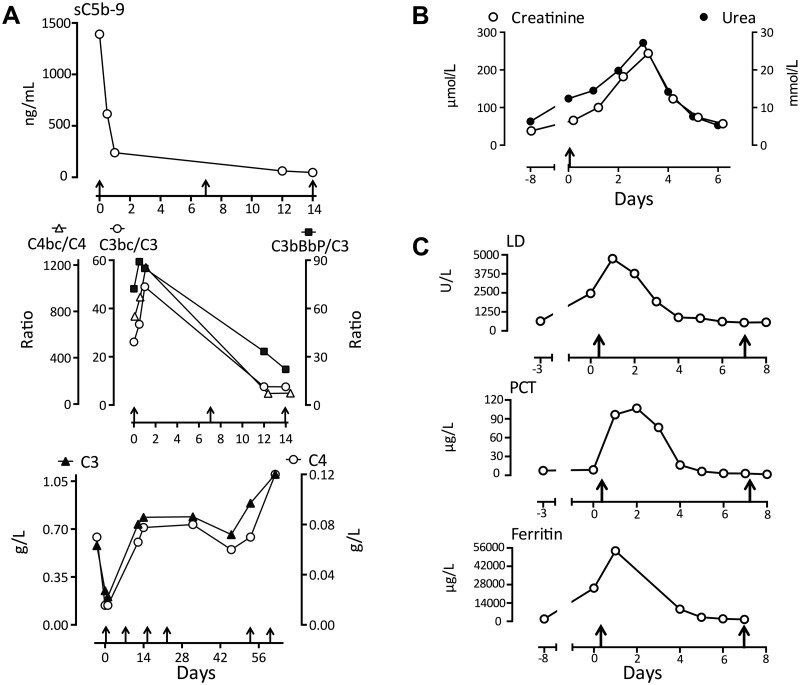
We present the case of a 34-year-old female patient diagnosed with APS after several miscarriages and deep venous thrombosis in 2004. The patient has repeatedly displayed positive lupus serology (ANA and ENA screening positive, anti-dsDNA = 50 U/l, ref <10) without clinical signs of lupus. Recently she was admitted with Staphylococcus aureus wound infection and sepsis. Biochemistry revealed anaemia, thrombocytopaenia, haemolysis, positive lupus anticoagulant (SCT = 2.6, ref <1.25; dRVVT = 3.1, ref <2.25) and high aPL (anti-β2-glycoprotein: IgG 62 U/l, IgM 535 U/l; anti-cardiolipin: IgG >410 U/l, IgM >470 U/l). Her condition deteriorated dramatically, with respiratory distress, circulatory collapse and cardiac arrest, requiring resuscitation and intensive care treatment. Renal function rapidly declined, with increasing creatinine and urea values (Fig. 1B). Eculizumab treatment was started, with remarkable clinical improvements within hours. After three days she did not require respiratory support and the kidney failure ceased (Fig. 1B). The level of sC5b-9 was exceptionally high initially, reflecting substantial intravascular complement activation, but decreased abruptly after administration of eculizumab (Fig. 1A). Importantly, the levels of activation product ratios declined and C3 and C4 increased simultaneously, consistent with a substantial reduction in complement activation upstream to C5 (Fig. 1A). Lactate dehydrogenase, procalcitonin and ferritin decreased abruptly after administration of eculizumab (Fig. 1C). Of particular note, eculizumab withdrawal led to a serious clinical relapse, with pain, tachycardia and tachypnoea, concomitant with decreased C3 and C4 (Fig. 1A). The triggering factor for this second episode was apparently a persistent wound infection. However, eculizumab rechallenge once again rapidly reversed the symptoms. This indicates that the effect was not coincidental to other treatment or spontaneous remission, but that there was a close relation between the degree of complement activation and the clinical course.
Fig. 1.
Complement activation in a patient with CAPS
(A) Complement activation products, native components, and the ratios thereof, were measured in the patient with CAPS during treatment with eculizumab (arrows along the x-axis refer to eculizumab infusion. Upper panel: sC5b-9; reference range <600 ng/ml. Middle panel: the ratio of complement activation products and their native components C4bc/C4 (reference range 28–140), C3bc/C3 (reference range 7–18) and C3bBbPb/C3 (reference range 18–48). Lower panel: C3 (reference range 0.5–1.3 g/l) and C4 (reference range 0.1–0.5 g/l). (B) Concentration of creatinine and urea (arrow: first infusion of eculizumab). (C) Concentration of lactate dehydrogenase (LD), procalcitonin (PCT) and ferritin (arrow: first infusion of eculizumab).
The present data elucidates the dynamics of complement activation and relates it to the clinical course of CAPS, suggesting complement activation to be an essential part in the pathophysiology and providing a rationale for therapeutic complement inhibition in CAPS. Low levels of C3 and C4 may indicate complement activation with consumption, but several other factors influence the protein levels. Thus, the measurement of complement activation products is required for documenting in vivo complement activation, and the ratio between an activation product and its parent protein is a sensitive indicator of activation. Markedly increased classical and/or lectin pathway activation was observed from the high C4bc/C4-ratio. Additionally, the substantial increased alternative pathway convertase (C3bBbP) and C3bBbP/C3-ratio reflect that the alternative pathway amplification loop was highly activated in CAPS. Finally, the terminal pathway was excessively activated, revealed by the high level of sC5b-9. The fact that eculizumab inhibits C5 indicates that the disease process is driven largely by terminal pathway activation with release of the potent pro-inflammatory anaphylatoxin C5a and formation of the C5b-9 complex. This leads to endothelial cell activation, including tissue factor expression, with subsequent serious fatal thrombus formation observed in CAPS [5]. Both C5a- and C5b-9 formation is efficiently inhibited by eculizumab [6]. In conclusion, complement inhibition should be regarded as an important and possibly life-saving medical intervention for patients with CAPS.
Funding: This study was financially supported by The Norwegian Council on Cardiovascular Disease, The Odd Fellow Foundation and the European Community’s Seventh Framework Programme under grant agreement no. 602699 (DIREKT).
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Pierangeli SS, Chen PP, Raschi E. et al. Antiphospholipid antibodies and the antiphospholipid syndrome: pathogenic mechanisms. Semin Thromb Hemost 2008;34:236–50. [DOI] [PubMed] [Google Scholar]
- 2.Cervera R, Font J, Gómez-Puerta JA. et al. Validation of the preliminary criteria for the classification of catastrophic antiphospholipid syndrome. Ann Rheum Dis 2005;64:1205–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapira I, Andrade D, Allen SL, Salmon JE. Brief Report: induction of sustained remission in recurrent catastrophic antiphospholipid syndrome via inhibition of terminal complement with eculizumab. Arthritis Rheum 2012;64:2719–23. [DOI] [PubMed] [Google Scholar]
- 4.Bergseth G, Ludviksen JK, Kirschfink M. et al. An international serum standard for application in assays to detect human complement activation products. Mol Immunol 2013;56:232–9. [DOI] [PubMed] [Google Scholar]
- 5.Vega-Ostertag ME, Pierangeli SS. Mechanisms of aPL-mediated thrombosis: effects of aPL on endothelium and platelets. Curr Rheumatol Rep 2007;9:190–7. [DOI] [PubMed] [Google Scholar]
- 6.Volokhina EB, Bergseth G, van de Kar NC, van den Heuvel LP, Mollnes TE. Eculizumab treatment efficiently prevents C5 cleavage without C5a generation in vivo. Blood 2015;126:278–9. [DOI] [PMC free article] [PubMed] [Google Scholar]