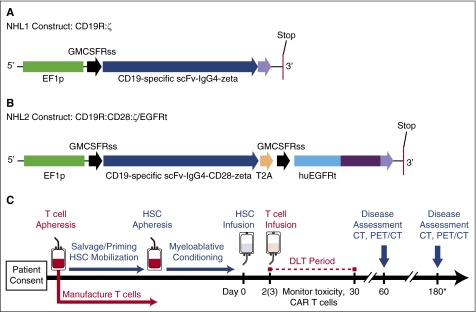
Figure 1.
Constructs and treatment schema for clinical trials. (A) The CD19R:ζ DNA sequence (optimized by GeneArt) that contains the CAR sequence consisting of the VH and VL gene segments (scFv) of the CD19-specific FMC63 monoclonal antibody (mAb), IgG4 hinge-CH2-CH3, and signaling domains CD3ζ (CD19CAR) was cloned into a self-inactivating lentiviral vector pHIV7 that has an EF-1 promoter. (B) In addition to CD19 scFv, the CD19R:CD28:ζ/EGFRt+ epHIV7 lentiviral construct also contains (1) the cytoplasmic domain of the CD3ζ chain; (2) the CD28 costimulatory domain, (3) the self-cleaving T2A sequence; and (4) the truncated EGFR sequence as indicated. The huEGFRt was synthesized by PCR splice overlap extension to fuse in frame the human granulocyte-macrophage–colony-stimulating factor receptor's leader peptide to domains III and IV and the transmembrane spanning components of huEGFR (base pairs, 1000-2004). This fusion product was then cloned into the epHIV7 vector (in which the cytomegalovirus promoter of pHIV7 was replaced with an EF-1 promoter) along with the CD19CAR and T2A sequences, and the final construct was confirmed by sequence analysis. (C) Leukapheresis for T-cell manufacturing may be drawn before or after cycles of salvage chemotherapy. Research participants received T-cell infusions on either day +2 or +3 after HSCT. *Disease assessments continue every 6 months until 2 years after HSCT (6, 12, 18, and 24 months). PET, positron emission tomography.