Abstract
Background:
Hospitals have attempted to reduce adverse drug events (ADEs) by investing in new technologies, but data regarding their efficacy are lacking.
Objectives:
This study evaluates the effects of the implementation of barcode medication administration (BCMA) and electronic medication administration record (eMAR) technology on the profile of ADEs in a hospital setting.
Methods:
We conducted a before-and-after study examining the effects of the implementation of BCMA and eMAR technology on the profile of ADEs at a 400-bed academic medical center by using incident reports. We compared reported ADEs in pre- and post-implementation periods of 5 months to determine whether there was a reduction in the rate of ADEs within medication use phases. We further examined the severity of errors and described changes in the distribution of types of errors.
Results:
A total of 775 electronic error-reporting system reports were included in this study: 397 (51%) in the pre-implementation period and 378 (49%) in the post-implementation period. The rate of ADEs significantly decreased from 0.26% to 0.20% after implementation of the technology (relative risk [RR], 0.78; 95% CI, 0.67–0.89). The rate of transcription errors decreased from 0.089% to 0.036% (RR, 0.40; 95% CI, 0.30–0.54), which was largely attributed to reduction of “wrong time” errors. The rate of administration errors was identical in both groups at 0.017% (RR, 0.98; 95% CI 0.58–1.66). The mean severity level of administration errors significantly decreased from 4.44 to 3.23 (p = .005).
Conclusion:
The implementation of eMAR and BCMA technology improved patient safety by decreasing the overall rate of ADEs and the rate of transcription errors. These technologies also reduced the harmful impact to patients caused by administration errors.
Keywords: adverse drug events, barcode medication administration technology, electronic medication administration record
BACKGROUND
Adverse drug events (ADEs) refer to errors that occur as a result of a mistake in delivering a drug to a patient, and they are a well-known problem in the medical community. ADEs occur with alarming frequency and are among the most common errors that affect patients in hospitals in the United States.1–3 Studies suggest that ADEs affect almost half of all hospitalized patients or possibly more.2,4,5 Although many of these errors do not reach the patient or cause harm, studies have estimated that up to 18% of patients may be affected by a serious ADE.2–4 Furthermore, ADEs are estimated to cost approximately $3.5 billion in the United States every year.6 Research on ADEs in the mid-1990s revealed the breadth of the problem, and it also offered some hope for improvement. A large percentage of ADEs were found to be caused by human error, and close to half of those that were most serious were found to be preventable.2,4
To systematically examine ADEs, researchers designated “medication-use phases” to better classify types of errors. These phases describe the process by which a medication is given to a patient in the hospital. It begins with (1) prescribing or writing a medication order, often by a medical doctor. Next is (2) transcription of the medication into a medication administration record (MAR), (3) dispensing by the pharmacy, and (4) administration of the medication to the patient. Some medications will then require (5) further monitoring (see Figure 1). Research has found that approximately one-third of ADEs take place in the initial prescription phase and another third occur at the administration phase.2 Those at the administration phase are more likely to reach the patient and therefore cause harm.2
Figure 1.
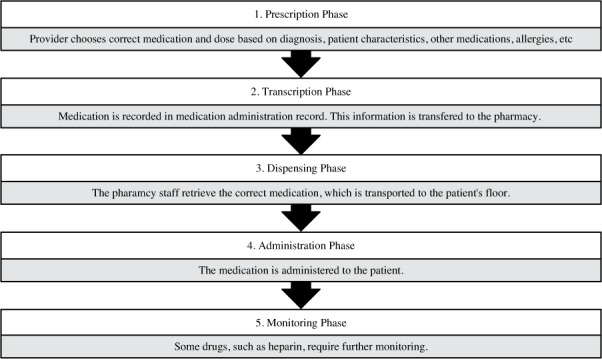
Description of the 5 phases of the medication-use process.
By focusing on error profiles within the medication-use process, developers have designed new types of health information technology (HIT) to reduce the number and severity of ADEs. Specifically, the electronic medication administration record (eMAR) was created to improve communication between the nurses and pharmacists and reduce transcription errors. Barcode medication administration (BCMA) technology was developed to decrease administration errors, and many hospitals utilize computerized physician order entry (CPOE) systems to decrease the number of prescription errors. The assumption is that such systems reduce the impact of ADEs, but the specifics of how they change the profile of errors remains somewhat obscure. HIT has been cited as particularly difficult to study due to the complexities of systems, variability within medical facilities, and the lack of controls during implementation.7 Reviews of the literature note a lack of consistent data to substantiate claims of effectiveness and improvement of patient outcomes, especially for commercially developed systems.8–10
Barcode Technology
The use of barcode technology has been identified as a way of improving the administration phase of medication use by confirming a patient's medication at bedside to ensure the 5 rights of medication administration – the correct drug, dose, time, route, and patient.11 Upon admission to the hospital, all patients are given a barcode wristband. Before administration of a medication, the nurse scans both the armband and a barcode on the medication. The BCMA technology confirms that the nurse is administering the medication correctly to the correct patient. If the medication or patient is incorrect, the nurse is notified, giving him or her a chance to correct the error prior to giving the medication.
BCMA technology can be an important tool in reducing the impact of ADEs, as administration errors are the most likely to reach the patient and therefore cause harm. By 2011, the technology had been implemented in 50% of hospitals in the United States and 58% of hospitals of similar size.12 Based on initial studies, BCMA technology appears to be cost-effective and reduces administration errors by over 50% and serious errors by up to 25%.13–16 It also decreases dispensing errors by changing workflow patterns, and it may improve medication administration documentation, billing, and public relations.17,18 However, these early studies were often performed on in-house developed systems. The results of later studies using commercial systems demonstrated an inconsistent decrease in errors.16,19 The system is not always used consistently; up to a tenth of medications are given without proper use of the technology.14,20 Furthermore, new errors specifically caused by BCMA technology have been reported.21,22 This has led to questions about effectiveness of commercially developed BCMA technology and its impact on patient care. Agency for Healthcare Research and Quality (AHRQ) considers commercial barcode systems an understudied technology that has not been conclusively shown to be effective in all circumstances.10
Medication Administration Check System
The hospital in which the study was conducted is a 415-bed academic medical center located in an urban center in New England. From 2008 to 2010, the departments of pharmacy and nursing informatics led the implementation of BCMA technology combined with an eMAR. This system is a commercial product developed and marketed by Siemens Corporation, and it is known as the Medication Administration Check (MAK) system. This change updated the hospital's system in several ways. First, the hospital had been using a handwritten paper MAR that was entirely separate from the pharmacy medication profile. The MAK system's eMAR combined the pharmacy and nursing records into a single system. Second, the switch from a paper system to an electronic system meant that the medication record could be updated and accessed in real-time. These 2 changes greatly improved communication between nursing and pharmacy staff within the hospital. Finally, the hospital implemented BCMA technology to decrease errors in administration of medications to patients. The purpose of this study is to compare the rate and type of ADEs at the hospital before and after the implementation of BCMA and eMAR technology.
METHODS
Approval for this study was granted through the health sciences campus institutional review board of the hospital. A retrospective comparison of ADEs pre and post BCMA and eMAR technology implementation was performed using hospital incident reports filed by hospital employees using DoctorQuality, a voluntary Web-based hospital-wide electronic error-reporting system (e-ERS). This system has been studied previously and used to describe a variety of adverse events across multiple institutions.3,23 Using pull-down response menus, reporters record the origin of event (discussed here as medication-use phase), type of event, and level of impact (discussed here as severity) and provide a description of the ADE. The BCMA and eMAR technology was implemented by the hospital between February 2009 and November 2010. All ADE reports were collected from a 5-month pre-implementation period of September 1, 2007 to February 29, 2008 and for 5 months in the post-implementation period from September 1, 2010 to March 1, 2011. System implementation was staggered by inpatient unit, and the study design allowed a minimum post-implementation wash-out period of 3 months; therefore departments that implemented the technology later than June 2010 were excluded from the study.
Reports were included in the study if they described an ADE occurring in adult inpatient departments within the study time periods. The adult psychiatry department was excluded because a new CPOE system was implemented in that department during the study's post-implementation time period, confounding the data. Reports from the research unit of the hospital were also excluded. A total of 1,183 reports were collected. Reports were then reviewed for accuracy; the medication-use phase, type of event, and event severity were compared with the description of the event to ensure the accuracy of the classifications. Those that did not match descriptions were re-classified. This review was conducted by a single individual, with expert oversight from the director of the department of pharmacy. Reports that did not have complete information to confirm their categorization were excluded from the study. In total, 142 reports were excluded from the pre-implementation period and 266 reports were excluded from the post-implementation period, leaving a total of 397 reports in the pre-implementation time period and 378 in the post-implementation time period.
ADEs in both periods were categorized in terms of medication-use phase, type, and severity. The 5 medication-use phases were prescription, transcription, dispensing, administration, and monitoring. The type of error could be defined as wrong patient, wrong drug, wrong dose, wrong route, wrong dosage form, wrong time, wrong rate, omitted, adverse drug event, dose given after drug discontinued, allergic to medication ordered, infiltration/extravasation, controlled substance procedure, monitoring – drug reaction, and other medication. The severity of the error was ranked between 1 and 8, with 1 being an error that did not reach a patient and 8 being an error that caused permanent harm or death to the patient. Errors were then further grouped to describe effects on the patient, with 1 to 2 not reaching the patient, 3 and 4 causing no harm, 5 to 7 causing temporary harm, and 8 causing permanent harm or death (see Figure 2).
Figure 2.
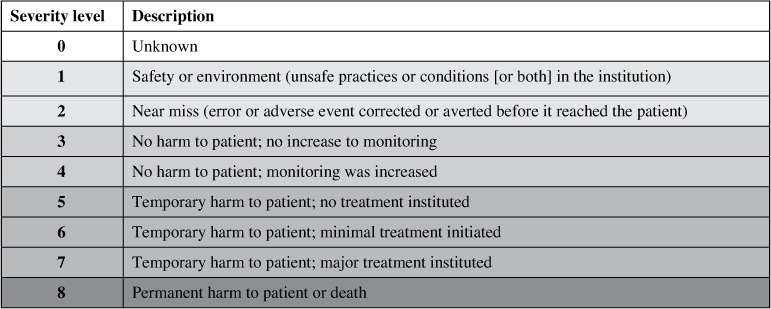
Severity levels of medication errors as defined by the electronic error reporting system.
The total number of medication orders placed in both study periods was obtained from the pharmacy department. Using this information, the rates of errors in the pre- and post-implementation periods were compared using rate ratios. Error rates within medication-use phases were then calculated, and rate ratios were calculated to determine if there was a change between pre- and post-implementation. To determine whether the severity of errors had changed, a 2-sided t test was used to compare the mean values within medication-use phases.
The characteristics of transcription errors and administration errors were examined and described. Within administration errors, those categorized as “controlled substance procedure” and “override for drug not in MAK” represent data collected for reporting purposes. These were excluded from the analysis, because they were identified exclusively by the new BCMA technology and therefore could not have been reported prior to its implementation. This prevented a falsely elevated administration error rate in the post-implementation period.
The statistical analysis was performed using IBM SPSS statistical software (IBM, Inc., Armonk, NY).
RESULTS
A total of 775 reports were analyzed in this study. In the pre-implementation period, there were 397 reports of ADEs and 150,000 medication orders recorded. The error rate in the control group was calculated to be 0.26%. In the post-implementation period, there were 378 reports and 184,000 medication orders, for an error rate of 0.20%. This was found to be a significant decrease in errors, with a rate ratio from the pre-implementation to the post-implementation periods of 0.78 (95% CI, 0.67–0.89).
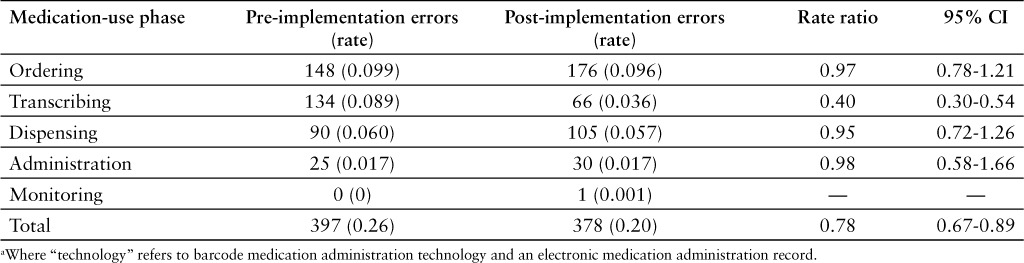
The number of error reports in the pre- and post-implementation groups in each medication-use phase was counted, and percentage of total errors in those groups was calculated (see Table 1, Figure 3). The number of transcription errors was found to be 134 in the pre-implementation period and 66 in the post-implementation period. The rate of transcription errors was calculated; there was a significant decrease from 0.089% to 0.036% (relative risk [RR], 0.40; 95% CI, 0.30–0.54). The number of administration error reports was 25 in the pre-implementation period and 30 in the post-implementation period. The error rate was calculated in both groups to be 0.017%, and there was no significant change (RR, 0.98; 95% CI 0.58–1.66). Ordering and distribution errors rates did not change significantly. There were no reports classified as monitoring errors in the pre-implementation group and only one in the post-implementation group, so these could not be compared (see Table 2).
Table 1.
Number of errors in each phase of the medication-use process: Pre- and post-technology a implementation periods
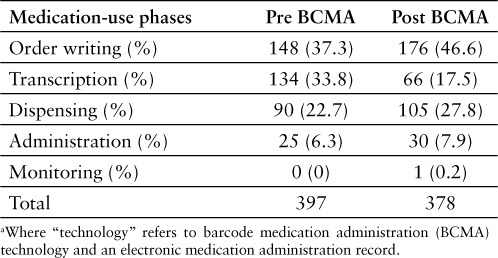
Figure 3.
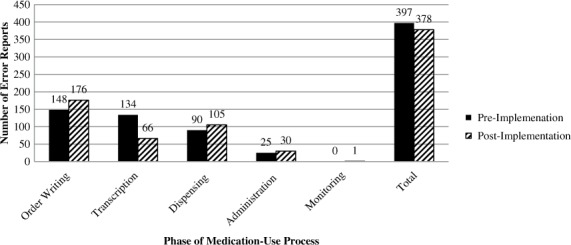
Number of errors in phases of medication use process during the pre- and post-technology implementation periods. “Technology” refers to barcode medication administration technology and an electronic medication administration record.
Table 2.
Rate of errors reported in each phase of the medication-use process: Pre- and post-technology a implementation periods
The severity level of errors reported in each medication-use phase is listed in Table 3. The large majority of errors in both pre- and post-implementation periods did not reach the patient (80% and 79%, respectively). Another 14% of errors in both groups reached the patient but did not cause harm. The percentage of errors that reached the patient and caused temporary harm was the same in both groups at 4%. There were no reports of ADEs causing permanent harm or death in either group. The change of the mean severity level is listed in Table 4. The mean severity level of administration errors decreased significantly from 4.44 to 3.23 (p = .005). The mean severity level did not change significantly in any other medication-use phase. Though the total number of administration errors that reached the patient increased from 17 to 22, the percentage of administration errors that caused harm to patients decreased from 36% to 20% (Figure 4).
Table 3.
Severity level of errors in each phase of the medication-use process: pre- and post-technology a implementation periods b
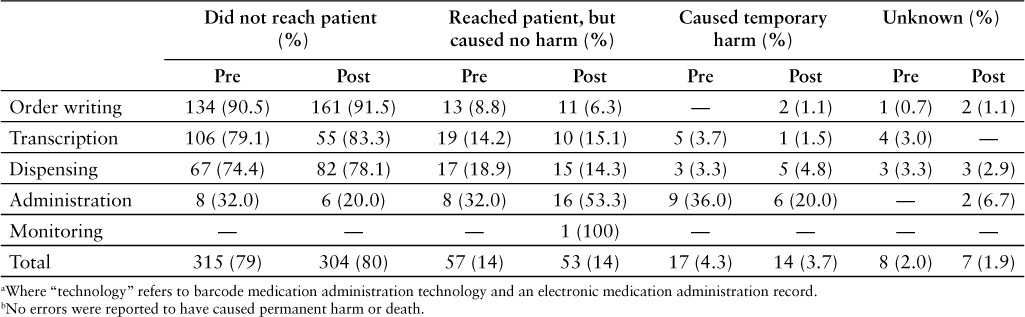
Table 4.
Mean severity level score within each phase of the medication-use process: Pre- and post-technology a implementation periods
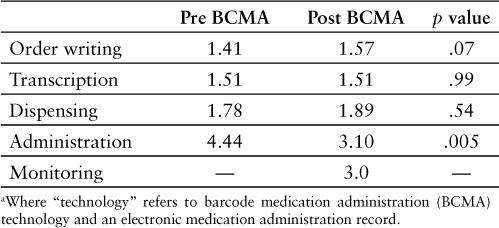
Figure 4.
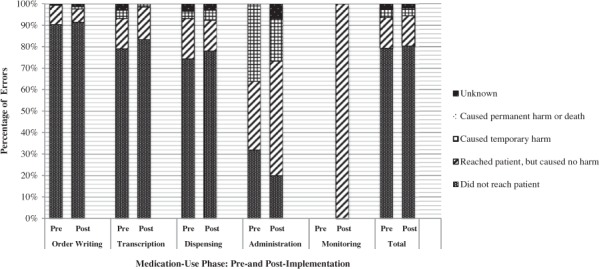
Percentage of errors in severity level categories in each medication phase during the pre- and post-technology implementation periods. “Technology” refers to barcode medication administration technology and an electronic medication administration record.
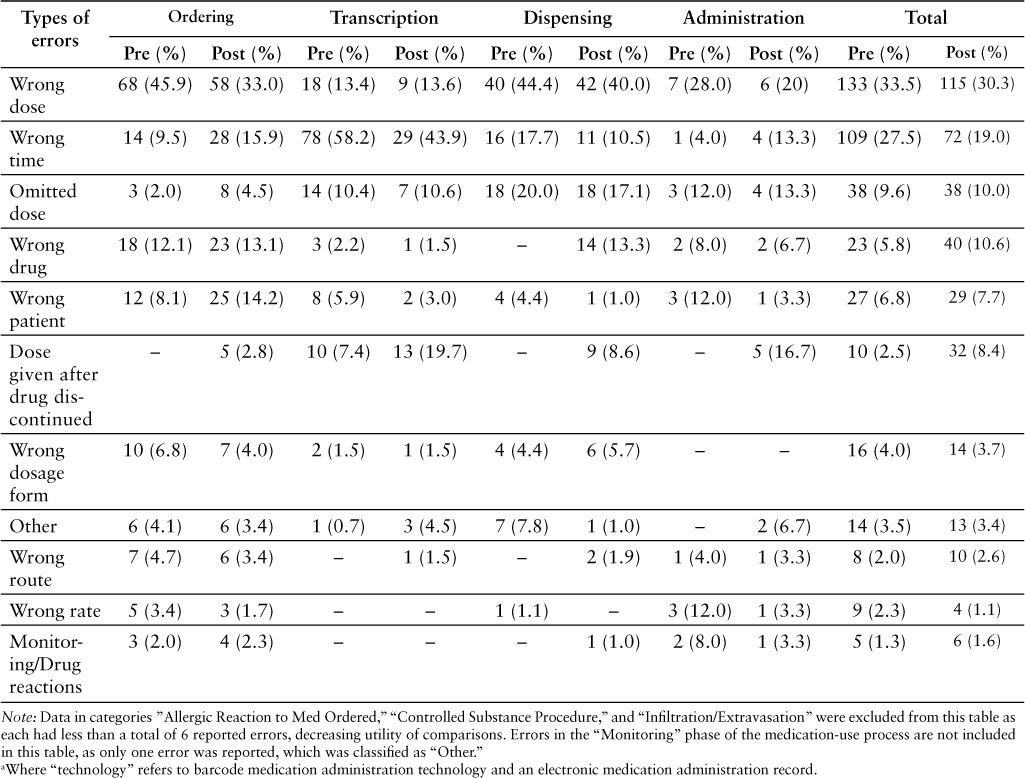
The types of errors within the medication phases in the pre- and post-implementation groups are described in Table 5. Within transcription errors, those considered “wrong time” made up the 78 of 134 (58%) transcription errors in the pre-implementation period. These decreased by 49, with a total of 29 in the post-implementation period, accounting for 44% of transcription errors. In administration errors, those described as “wrong dosage form” accounted for the largest number of errors in both the pre- and post-implementation periods (7 and 6, respectively). Types of errors that increased after BCMA implementation were those described as “dose given after drug discontinued” (0 to 5), “omitted” (3 to 4), and “wrong time” (1 to 4). The total number of errors categorized as “dose given after drug discontinued” in all medication-use phases increased from 10 to 32. This represents the category with the largest increase in errors after the implementation of the MAK technology.
Table 5.
Frequency of types of errors within each phase of the medication-use process: Pre- and post-technology a implementation periods
DISCUSSION
The introduction of the CPOE system, BCMA technology, and eMAR, as well as other technologies, has greatly changed medication delivery in hospitals. However, it remains unclear in studies to date how these technologies have changed the distribution of ADEs. This study attempted to answer a part of that question by exploring how the profile of errors changed after a hospital incorporated BCMA technology and an eMAR system. This study utilized data from a commercially developed system, which literature reviews note is limited.8,10
Our study showed that after implementation of BCMA and eMAR technologies, the reported medication error rate decreased by 20% (from 0.26% to 0.20%). It is worth noting that previous studies have found that incident reports may only capture approximately 1/200 of actual errors.24 Although incident reports grossly underestimate actual error rates, there is no apparent reason why reporting levels in our study would have decreased after the new technology was implemented other than a true reduction in errors. Therefore, we believe this suggests that the eMAR and BCMA technology accomplished the goal of reducing ADEs and improving patient safety.
We expected the rate of errors that occurred in the transcription phase to decrease due to the eMAR component of the MAK system and those in the administration phase to decrease due to the BCMA component. However we only saw this decrease in the transcription phase, which showed a statistically significant reduction of 60%. “Wrong time” transcription errors, in which a medication dose is late due to a delay in the transcription of the medication, decreased more than any other type of transcription error. This is not surprising, as we would expect technology that improves communication to decrease incidents of untimely transfer of information from the nursing station to the pharmacy. There was no significant change in the average severity level of these errors, and the percentage that reached patients and caused temporary harm remained low (3.7% to 1.5% of transcription errors). We found no transcription errors that caused permanent harm to patients, though other studies have documented this possibility.2
We initially hypothesized that the rate of administration phase errors would decrease. However, the rate of administration errors remained unchanged. This may be because the implementation of BCMA technology allowed for the identification of more administration errors, causing more to be reported. Even though the rate of reported administration errors did not decrease, the average severity level of these errors did decrease significantly. Only 6 errors caused harm to patients, a decrease from 9 prior to BCMA implementation. These data also support our hypothesis that the BCMA system reduced harm caused to patients from administration errors by allowing for early identification of the errors.
The types of errors that occurred in the administration phase were also examined. The sample size did not allow for an in-depth study of the changes in types of errors in the pre- and post-implementation periods. However, it is worth noting that of the 5 rights of medication administration, there was only an increase in error reports in the “wrong time” category. BCMA technology only gives alerts at point-of-care and therefore may not ensure that a medication is given on time. “Wrong time” errors are some of the most common types of administration errors, suggesting that this might be another area for intervention.5
There was an overall increase of errors classified as “dose given after drug discontinued.” This may be due to a reporting bias. Prior to implementation of the MAK system, the time of desired doses and precise time for the discontinuation of a medication were not always clearly identified in the paper MAR. The MAK system clearly identified these times, and therefore additional medication doses given after discontinuation were likely better detected. However, as it is not clear how these occur with an eMAR, this may still represent an important finding and could be an area of future investigation.
There are some limitations to our data. Due to the nature of the study, we were unable to randomize departments receiving the technology. Therefore, our control group and experimental group are separated by a period of time. Though we were not able to identify any large-scale changes to pharmacy or hospital policy during this time, staffing changes, reporting initiatives, and other events could have potential impacts on our data.
The MAK system implementation changed the medication use system in multiple ways, as described above. This has the possibility of causing confusion between the effects of the separate components of the MAK system. We assume migration to an eMAR is to credit for the decrease of transcription errors, while previous studies have shown BCMA technology impacts administration errors and possibly dispensing errors, but no studies have shown a change in transcription errors. There is no way to be certain that the 2 components would not have an effect on one another. Furthermore, the MAK system also incorporates identification of errors. This likely led to a reporting bias, especially in administration errors.
The data that we used are from incident reports, which is a small representation of actual errors; this may also bias toward certain types of errors.24 We attempted to decrease this bias in our study by avoiding comparisons between errors in different medication-use phases and types of errors within the pre- or post-implementation groups. An example of the effect of using incident reports can be seen in the distribution of the error reports in the pre- and post-implementation periods and how they differ from that of other studies. The literature shows approximately one-third of errors occur in the order writing phase, with another third in the administration phase.2 In our study, errors were skewed more toward the early stages, with only 7% of reports describing errors in the administration phase. This difference is likely due to reporting bias, as earlier errors are discovered at higher rates than errors later in the medication use process and therefore represent a greater number of reports in the e-ERS.2
Incident reports are also reliant on the initial reporter in making an accurate report. Reports may be incorrect from a lack of knowledge or bias by reporters. Continued support of the use of the e-ERS and training on reporting errors could improve the accuracy of these reports.
The use of incident reports has strengths as well. It allows for a large amount of data to be collected and analyzed quickly. It likely overrepresents clinically significant errors, which therefore may help identify parts of the medication use system where intervention could have the greatest impact for patients, some of which have been previously identified.24,25 For example, in our data, we found 18 incidents that specifically mentioned the transfer of a patient between services. Other studies have noted the transfers of patients to be vulnerable areas in the system, and such transition periods are an important area for future study and improvement.26,27 We also hope to explore the use of overrides of the MAK system. Through data collected directly by the system, we hope to identify the inappropriate overrides of the barcode technology. This will help us identify further weak areas in the system, which can lead to improvements and a further reduction of harm caused to patients.
This examination of incident reports has helped add to our knowledge concerning HIT, specifically the BCMA system and eMAR, and its use in reducing ADEs in hospitals. The examination of transcription errors showed a significant decrease in reported errors, likely due to the implementation of the eMAR. Though we did not find a significant decrease in rates of administration errors due to the BCMA technology, we did see a significant decrease in the harm caused to patients. This is an important finding as it suggests that, even if the true rate of administration errors did not decrease, BCMA technology may still play an important clinical role in reducing iatrogenic harm to patients in the hospital.
ACKNOWLEDGMENTS
We thank Tufts University Department of Public Health for their support of this study with mentorship and resources to perform the statistical analysis.
Footnotes
Internal Medicine Intern, Beth Israel Deaconess Medical Center, Boston, Massachusetts
Director of Pharmacy, Tufts Medical Center, Boston, Massachusetts
Chief of Internal Medicine and Primary Care, Tufts Medical Center, Boston, Massachusetts
Tufts University, Boston, Massachusetts
Physician-in-Chief, Department of Medicine, Tufts Medical Center, Boston, Massachusetts.
REFERENCES
- 1.Kohn LT, Corrigan JM, Donaldson MS, editors. To Err Is Human: Building a Safer Health System. Washington, DC: National Academies Press; 1999. [PubMed] [Google Scholar]
- 2.Bates DW, Cullen DJ, Laird N et al. Incidence of adverse drug events and potential adverse drug events: Implications for prevention. JAMA. 1995;274:29–34. (ADE Prevention Study Group) [PubMed] [Google Scholar]
- 3.Milch CE, Salem DN, Pauker SG, Lundquist TG, Kumar S, Chen J. Voluntary electronic reporting of medical errors and adverse events: An analysis of 92,547 reports from 26 acute care hospitals. J Gen Intern Med. 2006;21:165–170. doi: 10.1111/j.1525-1497.2006.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews LB, Stocking C, Krizek T et al. An alternative strategy for studying adverse events in medical care. Lancet. 1997;349:309–313. doi: 10.1016/S0140-6736(96)08268-2. [DOI] [PubMed] [Google Scholar]
- 5.Lisby M, Nielsen LP, Mainz J. Errors in the medication process: Frequency, type, and potential. Int J Qual Health Care. 2005;17(1):15–22. doi: 10.1093/intqhc/mzi015. [DOI] [PubMed] [Google Scholar]
- 6.Aspden P, Wolcott JA, Bootman JL, Cronenwett LR, editors. Preventing Medication Errors: Quality Chasm Series. Washington DC: National Academies Press; 2007. [Google Scholar]
- 7.Greenhalgh T, Russell J. Why do evaluations of eHealth programs fail? An alternative set of guiding principles. PLoS Med. 2010;7(11):e1000360. doi: 10.1371/journal.pmed.1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black AD, Car J, Pagliari C, Anandan C, Cresswell K et al. The impact of ehealth on the quality and safety of health care: A systematic overview. PLoS Med. 2011;8(1):e1000387. doi: 10.1371/journal.pmed.1000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oren E, Shaffer E, Guglielmo J. Impact of emerging technologies on medication errors and adverse drug events. Am J Health Syst Pharm. 2003;60:1447–1458. doi: 10.1093/ajhp/60.14.1447. [DOI] [PubMed] [Google Scholar]
- 10.McKibbon KA, Lokker C, Handler SM Enabling Medication Management Through Health Information Technology. Rockville MD: Agency for Healthcare Research and Quality; 2011. pp. 1–925. Evidence Report/Technology Assessment. No. 201. [Google Scholar]
- 11.Shaw A. Pharmacy Intervention in the Medication-Use Process: The Role of Pharmacists in Improving Patient Safety. Den Haag, Netherlands: International Pharmaceutical Federation; 2009. https://www.fip.org/files/fip/Patient%20Safety/PatientSafetyAdvidShah.pdf. [Google Scholar]
- 12.Pedersen CA, Schneider P, Scheckelhoff D. ASHP national survey of pharmacy practice in hospital settings: Dispensing and administration – 2011. Am J Health Syst Pharm. 2012;69:768–785. doi: 10.2146/ajhp110735. [DOI] [PubMed] [Google Scholar]
- 13.Maviglia SM, Yoo JY, Franz C et al. Cost-benefit analysis of a hospital pharmacy bar code solution. Arch Intern Med. 2007;167:788–794. doi: 10.1001/archinte.167.8.788. [DOI] [PubMed] [Google Scholar]
- 14.Paoletti RD, Suess TM, Lesko MG et al. Using bar-code technology and medication observation methodology for safer medication administration. Am J Health Syst Pharm. 2007;64:536–543. doi: 10.2146/ajhp060140. [DOI] [PubMed] [Google Scholar]
- 15.Sakowski J, Leonard T, Colburn S et al. Using a bar-coded medication administration system to prevent medication errors. Am J Health Syst Pharm. 2005;62:2619–2625. doi: 10.2146/ajhp050138. [DOI] [PubMed] [Google Scholar]
- 16.Cochran GL, Jones KJ et al. Errors prevented by and associated with bar-code administration systems. Jt Comm J Qual Patient Saf. 2007;33:293–301. 245. doi: 10.1016/s1553-7250(07)33034-1. [DOI] [PubMed] [Google Scholar]
- 17.Poon EG, Cina JL, Churchill W et al. Medication dispensing errors and potential adverse drug events before and after implementing bar code technology in the pharmacy. Ann Intern Med. 2006;145:426–434. doi: 10.7326/0003-4819-145-6-200609190-00006. [DOI] [PubMed] [Google Scholar]
- 18.Cummings J, Bush P, Smith D, Matuszewski K. Bar-coding medication administration overview and consensus recommendations. Am J Health Syst Pharm. 2005;62:2626–2629. doi: 10.2146/ajhp050222. [DOI] [PubMed] [Google Scholar]
- 19.Helmans Pieter, Wargel Lindsay, Daniels Charles. Effect of bar-code-assisted medication administration on medication administration errors and accuracy in multiple patient care areas. Am J Health Syst Pharm. 2009;66:1202–1210. doi: 10.2146/ajhp080357. [DOI] [PubMed] [Google Scholar]
- 20.Wakefield DS, Ward MM, Loes JL, O'Brien J. A network collaboration implementing technology to improve medication dispensing and administration in critical access hospitals. J Am Med Inform Assoc. 2010;17:584–587. doi: 10.1136/jamia.2010.004267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medication errors occurring with the use of bar-code administration technology. Pa Patient Saf Advis. 2008;5(4):122–126. [Google Scholar]
- 22.Koppel R, Wetterneck T, Telles JL et al. Workarounds to barcode medication administration systems: Their occurrences, causes, and threats to patient safety. J Am Med Inform Assoc. 2008;15:408–423. doi: 10.1197/jamia.M2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amori R, Pittas A et al. Inpatient medical errors involving glucose-lowering medications and their impact on patients: Review of 2,598 incidents from a voluntary electronic error-reporting database. Endocr Pract. 2008;14(5):535–542. doi: 10.4158/EP.14.5.535. [DOI] [PubMed] [Google Scholar]
- 24.Flynn EA, Barker KN, Pepper GA et al. Comparison of methods for detecting medication errors in 36 hospitals and skilled-nursing facilities. Am J Health Syst Pharm. 2002;59:436–446. doi: 10.1093/ajhp/59.5.436. [DOI] [PubMed] [Google Scholar]
- 25.US Pharmacopeia Revision Bulletin. Physical environments that promote safe use. General Chapter <1066>. October 1, 2010. www.usp.org/sites/default/files/usp_pdf/EN/USPNF/gc1066PhysicalEnvironments.pdf. Accessed December 8, 2013.
- 26.Bobb A, Gleason K et al. The epidemiology of prescribing errors: The potential impact of computerized prescriber order entry. Arch Intern Med. 2004;164:785–792. doi: 10.1001/archinte.164.7.785. [DOI] [PubMed] [Google Scholar]
- 27.Kripalani S, Roumie C et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge. Ann Intern Med. 2012;157:1–10. doi: 10.7326/0003-4819-157-1-201207030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]


